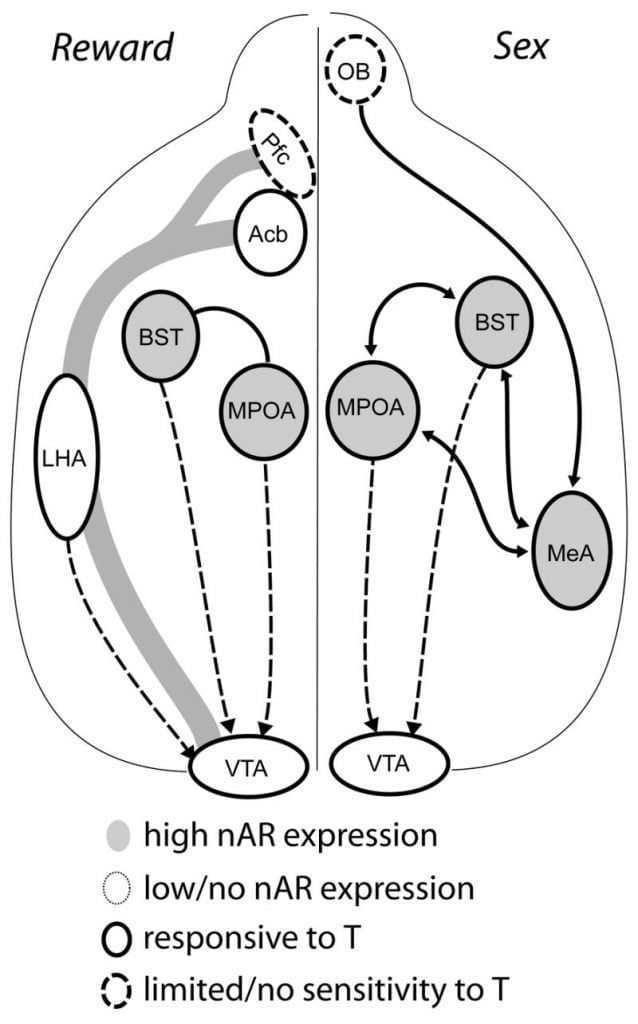
COMMENTS: Excellent review of almost all the relevant research on androgen receptors, dopamine and sexual function. Fantastic drawing the the hypothalamus-reward circuit interrelationships.
Horm Behav. 2008 May; 53(5): 647–658.
Published online 2008 February 13. doi: 10.1016/j.yhbeh.2008.01.010
Abstract
Adolescence is associated with increases in pleasure-seeking behaviors, which, in turn, are shaped by the pubertal activation of the hypothalamo-pituitary-gonadal axis. In animal models of naturally rewarding behaviors, such as sex, testicular androgens contribute to the development and expression of the behavior in males. To effect behavioral maturation, the brain undergoes significant remodeling during adolescence, and many of the changes are likewise sensitive to androgens, presumably acting through androgen receptors (AR). Given the delicate interaction of gonadal hormones and brain development, it is no surprise that disruption of hormone levels during this sensitive period significantly alters adolescent and adult behaviors. In male hamsters, exposure to testosterone during adolescence is required for normal expression of adult sexual behavior. Males deprived of androgens during puberty display sustained deficits in mating. Conversely, androgens alone are not sufficient to induce mating in prepubertal males, even though brain AR are present before puberty. In this context, wide-spread use of anabolic-androgenic steroids (AAS) during adolescence is a significant concern. AAS abuse has the potential to alter both the timing and the levels of androgens in adolescent males. In hamsters, adolescent AAS exposure increases aggression, and causes lasting changes in neurotransmitter systems. In addition, AAS are themselves reinforcing, as demonstrated by self-administration of testosterone and other AAS. However, recent evidence suggests that the reinforcing effects of androgens may not require classical AR. Therefore, further examination of interactions between androgens and rewarding behaviors in the adolescent brain is required for a better understanding of AAS abuse.
Overview
Adolescence awakens the brain to both pleasure and risk. In human teenagers, this frequently takes the form of experimentation with drugs and sex. In the United States, the median age for first intercourse in males is 16.4 years, and 65% have had intercourse by 12th grade (Kaiser Family Foundation, 2005). Likewise, this population has the highest rates of illicit drug use in the United States. According to the 2004 National Survey on Drug Use and Health, 38% of men ages 18–25 used an illicit drug in the past year (SAMHSA/OAS, 2005). Moreover, 31% of teen boys used drugs or alcohol during their last sexual encounter (Kaiser Family Foundation, 2005). In addition, adolescence is a pivotal time in the etiology of certain psychopathologies, such as depression, anxiety, disordered eating, and conduct disorder. We posit that the pubertal secretion of gonadal hormones, their activation of steroid receptors in the brain, and the interaction between hormone and experience on adolescent brain development contribute to the behavioral changes seen during adolescence.
Our goal here is to review the evidence that gonadal androgens mediate the adolescent maturation and adult performance of motivated behaviors, as well as the rewarding properties of these behaviors. We also present evidence that testosterone itself is rewarding, which likely contributes to maturational changes in motivated behaviors during adolescence, when testosterone levels soar. The focus of this paper is on our studies of neural circuits underlying male sexual behavior, particularly in the Syrian hamster, with special emphasis on the interaction between testosterone and dopamine (DA). We propose that pubertal androgens have both transient and long-term effects on reward circuits and motivated behavior. We further hypothesize that supplementation with exogenous androgens in the form of anabolic-androgenic steroids (AAS) augments the normal influences of pubertal androgens, thereby adversely affecting adolescent development of brain and behavior.
Adolescence as a sensitive period for brain development
Ultimately, the brain is both a trigger and a target for androgen action during adolescence. In young boys (<12 years) and young hamsters (<28 days of age), circulating androgens and gonadotropins are at basal levels. As secretion of luteinizing hormone from the anterior pituitary gland rises in response to hypothalamic gonadotropin-releasing hormone, circulating testosterone concentrations increase significantly. This occurs by Tanner stage II/III (14 years) in boys, and by 28 days of age in hamsters. By the time boys reach Tanner stage IV/V (ca. 16 years of age) or when hamsters are 50–60 days of age, endogenous testosterone is within the adult male range. Pubertal hormone secretion coincides with the period of adolescence, which takes place from approximately 12 to 20 years of age in humans. Pubertal hormones act not only on peripheral tissues to cause the appearance of secondary sex characteristics that are the overt signs of puberty, but they also act centrally to influence both the remodeling of the adolescent brain and behavioral maturation. Furthermore, the physiologic and neurologic changes brought about by pubertal hormones lead to significant changes in an individual’s experience, which can itself profoundly alter the course of brain development. Thus, the pubertal increase in sex steroid hormones, driven by developmentally timed maturation of the reproductive neuroendocrine axis, in turn shapes adolescent behavioral development via both direct and indirect influences on the nervous system.
Human adolescence is now recognized as a major and dynamic period of neural development during which behavioral circuits are remodeled and refined. Although the brain of a 5-year-old child is already 90% of its adult size (Dekaban, 1978), significant remodeling is still to come. This concept was kindled by research in both humans and animals documenting that many of the basic developmental processes occurring during perinatal brain development are recapitulated during adolescence. These processes include neurogenesis (Eckenhoff and Rakic, 1988; He and Crews, 2007; Pinos, Collado, Rodriguez-Zafra, Rodriguez, Segovia, and Guillamon, 2001; Rankin, Partlow, McCurdy, Giles, and Fisher, 2003), programmed cell death (Nunez, Lauschke, and Juraska, 2001; Nunez, Sodhi, and Juraska, 2002), elaboration and pruning of dendritic arborizations and synapses (Andersen, Rutstein, Benzo, Hostetter, and Teicher, 1997; Huttenlocher and Dabholkar, 1997; Lenroot and Giedd, 2006; Sowell, Thompson, Leonard, Welcome, Kan, and Toga, 2004), myelination (Benes, Turtle, Khan, and Farol, 1994; Paus, Collins, Evans, Leonard, Pike, and Zijdenbos, 2001; Sowell, Thompson, Tessner, and Toga, 2001), and sexual differentiation (Chung, De Vries, and Swaab, 2002; Davis, Shryne, and Gorski, 1996; Nunez et al., 2001). Thus, the developmental trajectory of the postnatal brain is not linear, but is instead characterized by an adolescent burst of rapid change and involves both progressive and regressive events. As any developmental biologist knows, periods of rapid developmental change signal heightened sensitivity and vulnerability to both experience-dependent change and to adverse consequences of perturbation and insult, and there is no reason to think that human adolescent brain development is any exception (Andersen, 2003; Spear, 2000). Thus, perturbations in the timing of pubertal hormone influences on the adolescent brain would be predicted to have long-lasting consequences for adult behavior.
Androgens and Neural Circuits for Motivated Behavior
Because adolescence is a transient and dynamic phase of development, it would be difficult to evaluate the adolescent brain and behavior in isolation. Instead, to appreciate the unique character of adolescence, it is helpful to contrast it with the brain and behavior of mature adults. Thus, with the focus of this paper on male sexual behavior and reward, it is important here to introduce the neural circuits for copulation and sexual motivation in adult males, including the role of gonadal steroid hormones in behavioral activation and the distribution of receptors for androgens (AR) and estrogens (ER).
AR are present in cell groups that form the neural circuits mediating rewarding social behaviors, such as sex. Furthermore, brain AR are expressed before puberty in hamsters and are upregulated by androgens in both juvenile and adult males (Kashon, Hayes, Shek, and Sisk, 1995; Meek, Romeo, Novak, and Sisk, 1997). In rodent brain, there is substantial overlap in the distribution of AR and ER (Wood and Newman, 1995), and aromatase (Celotti, Negri-Cesi, and Poletti, 1997), including both α and β forms of the estrogen receptor (Shughrue, Lane, and Merchenthaler, 1997). Upon binding to ligand, “classical” AR and ER function as transcription factors to induce transcription and synthesis of new proteins. Not surprisingly, these effects follow a relatively slow time-course, with a delayed onset of action. Steroid stimulation of male hamster sexual behavior (Noble and Alsum, 1975) is consistent with actions through classical genomic actions. For example, 2 weeks of steroid exposure is required to restore mating in long-term castrates. More recent studies in rats have also demonstrated rapid cellular effects of androgens in brain regions that possess few classical receptors (Mermelstein, Becker, and Surmeier, 1996). These steroid actions are thought to be mediated by non-genomic receptors. Whereas the distribution of classical AR and ER in the hamster brain is relatively restricted (Wood and Swann, 1999), the potential brain targets for non-genomic androgen action are much broader.
The medial preoptic area (MPOA) plays a central role in copulation in males from goldfish to humans (reviewed in Hull, Wood, and McKenna, 2006). Moreover, the hamster MPOA transduces gonadal steroid hormones via abundant AR and ER, and testosterone implants in MPOA are sufficient to restore sexual activity in long-term castrates (Wood and Swann, 1999). In male rats, gonadal steroids act in the MPOA to regulate basal DA release (Putnam, Sato, and Hull, 2003) and stimulate mating (Hull, Du, Lorrain, and Matuszewich, 1995). Initially, there is a modest increase in DA when a female is presented behind a screen. During copulation, MPOA DA increases further (+50% of baseline), and this effect requires androgens (Hull et al., 1995; Putnam et al., 2003). Not surprisingly, in castrated males that do not mate, MPOA DA does not increase (Hull et al., 1995). It is somewhat difficult to interpret this result, since the lack of DA release is confounded by the absence of sexual activity. However, DA release in MPOA correlates with the loss of mating in short-term castrates (Hull et al., 1995), and with testosterone-induced restoration of sexual activity in long-term castrates (Du, Lorrain, and Hull, 1998; Putnam, Du, Sato, and Hull, 2001).
Within the rodent MPOA, the androgenic and estrogenic metabolites of testosterone play specific roles in the regulation of mating (Putnam et al., 2003; Putnam, Sato, Riolo, and Hull, 2005). The latency to initiate copulation (mount or intromit) is one measure of sexual motivation. The latency to sexual activity is sensitive to estrogens, through maintenance of MPOA nitric oxide synthase, which in turn, maintains basal DA levels. Estrogen-treated castrates show high basal DA levels, which strongly correlate with the ability to initiate copulation. However, they fail to show female- and copulation-induced increases in DA release, which strongly correlate with sexual performance. Consequently, their sexual performance is below intact levels. On the other hand, castrates treated with non-aromatizable androgen alone do not show elevated basal DA levels, and they fail to initiate copulation. For normal sexual performance, therefore, both estrogens and androgens are required. Sexual performance is usually expressed as frequency measures of mounts, intromissions, and ejaculations. Only when both estrogens and androgens are replaced, do castrated males exhibit elevated DA levels (and shorter latency measures) and female- and copulation-induced DA increases (and increased frequency measures). In this manner, estrogens in MPOA contribute to sexual motivation, and both estrogens and androgens to sexual performance.
Although testosterone is necessary for MPOA DA release during male copulatory behavior and for mating itself, neither testosterone nor mating alone can elicit DA in MPOA. Instead, chemosensory cues from conspecific females are also required for DA release in MPOA. In rodents, chemosensory stimuli are the primary sensory modality to initiate male sexual behavior (Fig. 1). Chemosensory cues are transmitted from the olfactory bulbs to MPOA via the medial amygdaloid nucleus and the bed nucleus of the stria terminalis, structures with abundant AR and ER (Wood and Swann, 1999). To determine the role of chemosensory cues in mating-induced DA, we measured MPOA DA during mating in gonad-intact male hamsters with unilateral olfactory bulbectomy (UBx, Triemstra, Nagatani, and Wood, 2005). Although bilateral removal of the olfactory bulbs eliminates sexual activity and MPOA DA release, unilateral bulbectomy does not interfere with mating. In this study, copulation induced MPOA DA release when measured contralateral to the lesioned olfactory bulb, but not in the ipsilateral hemisphere (Fig. 2). Similar results were observed in male rats with lesions of the medial amygdala (Dominguez, Riolo, Xu, and Hull, 2001). In a related study, chemical stimulation of the medial amygdala in rats induced MPOA DA release equivalent to that during copulation (Dominguez and Hull, 2001). Taken together, these data suggest that testosterone creates a permissive environment that allows external sensory stimuli to reach MPOA and induce DA release during copulation.

Fig. 2
Ultimately, sexual behavior and other natural rewards activate neural reward pathways. The mesocorticolimbic DA circuit consists of the ventral tegmental area (VTA), nucleus accumbens (Acb), and prefrontal cortex (Pfc). Dopamine cell bodies residing in the VTA project rostrally to the Acb and Pfc (Koob and Nestler, 1997). In rats, DA is released into Acb during sex (Pfaus, Damsma, Nomikos, Wenkstern, Blaha, Phillips, and Fibiger, 1990). Many drugs of abuse also act in the mesolimbic DA system to increase DA release (amphetamines) or inhibit DA reuptake (cocaine, Di Chiara and Imperato, 1988), thus reinforcing their addictive properties. In this manner, testosterone has the potential to affect the release of DA in Acb both through its enhancement of sexual behavior, and through its actions as a drug of abuse (see below).
Current evidence suggests that the mesocorticolimbic DA system matures during adolescence. Acb DA fiber densities increase dramatically during adolescence in gerbils, suggesting that significant maturation of VTA dopaminergic projections to the Acb occurs during the adolescent period (Lesting, Neddens, and Teuchert-Noodt, 2005). Furthermore, dopaminergic input to GABA (γ-aminobutyric acid)-ergic cells in the rat medial prefrontal cortex is enriched and modulated by serotonergic systems during pubertal development (Benes, Taylor, and Cunningham, 2000), and manipulation of androgens in adult rats leads to changes in dopaminergic axon density within prefrontal cortex (Kritzer, 2003). The Pfc, Acb, and the VTA have few AR or ER, although ERβ is present in the VTA (Shughrue et al., 1997). Therefore, it seems likely that androgens affect the mesocorticolimbic DA system through androgen-sensitive afferents or through ERβ in the VTA as in hypothalamus (Handa et al., this issue). Our data show that androgen-sensitive cells in male hamsters project to the VTA from structures associated with steroid-sensitive behaviors. For example, both the MPOA and the bed nucleus of stria terminalis (BST) contain a large number of AR-positive cells projecting to the VTA (Sato and Wood, 2006). The ventral pallidum, the major Acb efferent target (Zahm and Heimer, 1990), also contains many AR-positive cells projecting to the VTA. These projections provide an opportunity for androgens to modify the activity of the mesocorticolimbic DA system.
Steroid-dependent organization of behavior during adolescence
The traditional view of hormone action on adolescent behavior is based on activational effects of steroid hormones, which refer to the ability of steroids to facilitate behavior in specific social contexts by action within target cells in the neural circuits underlying behavior. Activational effects are transient in the sense that they come and go with the presence and absence of hormone, and they are typically associated with the expression of adult behavior. In contrast, organizational effects refer to the ability of steroids to sculpt nervous system structure during development. Structural organization is permanent, persists beyond the period of exposure to hormone, and determines neural and behavioral responses to steroids in adulthood. Our understanding of the developmental relationship between organizational and activational effects of steroid hormones has evolved over the past 50 years. Phoenix and colleagues first proposed that adult behavioral (activational) responses to steroid hormones are programmed (organized) by steroid hormones during a maximally sensitive period of perinatal development (Phoenix, Goy, Gerall, and Young, 1959). Later, Scott and colleagues laid the theoretical groundwork for the existence of multiple sensitive periods for the progressive organization of the nervous system, and noted that sensitive periods are most likely to occur during periods of rapid developmental change (1974). Subsequently, Arnold and Breedlove pointed out that steroid-dependent organization of the brain can occur outside of sensitive periods of development (Arnold and Breedlove, 1985). Over the past 15 years, research employing a variety of animal models and behavioral systems makes it clear that the adolescent brain is sensitive to both activational and organization effects of gonadal steroids (reviewed in Sisk and Zehr, 2005). And, like other periods of rapid developmental change, adolescence represents a defined window of opportunity for steroid-dependent brain remodeling.
Our work using the hamster as an animal model provides evidence that male social behaviors are modified by steroids during adolescence (Schulz, Menard, Smith, Albers, and Sisk, 2006; Schulz and Sisk, 2006). Before puberty, testosterone treatment cannot activate sexual behavior in hamsters, suggesting that maturational processes that render neural circuits susceptible to activation or organization by steroid hormones have not yet occurred (Meek et al., 1997; Romeo, Richardson, and Sisk, 2002a). Conversely, while the overt expression of male reproductive behavior in adulthood does not absolutely require the presence of gonadal steroids during adolescence, the maximal expression of behavior does. Comparing masculine reproductive behavior in males castrated either prepubertally (NoT@P) or postpubertally (T@P) and then treated with testosterone in adulthood, prepubertal NoT@P castrates have at least a 50% deficit in masculine behavior compared with males castrated after adolescence (Fig. 3, Schulz, Richardson, Zehr, Osetek, Menard, and Sisk, 2004). Moreover, deficits in reproductive behavior are long-lasting, and cannot be overcome either by prolonged testosterone treatment or by sexual experience in adulthood (Schulz et al., 2004). Similarly, after treatment with estrogen and progesterone, NoT@P males display shorter lordosis latencies and longer lordosis durations than males castrated as adults (Schulz et al., 2004), suggesting that prepubertal castrates are less defeminized than the males exposed to pubertal testosterone.

Fig. 3
It may be that NoT@P males suffer from decreased sexual motivation. One way to address this question is to compare the latencies to engage in both ano-genital investigation (AGI) and mounting between males gonadectomized before (NoT@P) and after puberty (T@P). If sexual motivation is dependent on gonadal hormone exposure during adolescence, we would predict longer latencies to engage in sexual behavior in NoT@P males. Indeed, with repeated exposure to estrous females, NoT@P males take longer to begin AGI and mounting compared with T@P males (Fig. 4). Thus, in addition to organizing aspects of sexual performance, it appears that pubertal hormones also organize the rewarding aspects of sexual behavior. In support of this possibility, central administration of the DA agonist apomorphine in adulthood restores mounting behavior of NoT@P males to adult-typical levels, suggesting that testosterone during adolescence normally organizes dopaminergic neural circuits (Salas-Ramirez, Montalto, and Sisk, 2006). Nonetheless, many interesting questions remain. Would a NoT@P male barpress for an estrous female or develop a conditioned place preference for a mating location? Future research will explore the role of pubertal hormones in organizing sexual motivation and sexual performance.

Fig. 4
Anogenital investigation (AGI) latencies and durations exhibited by male hamsters gonadectomized before puberty (NoT@P) or after puberty (T@P). All males were testosterone-primed in adulthood 7 wk after gonadectomy and one week prior to the first behavior test. A. T@P males showed similar AGI latencies across the three tests with an estrous female, whereas NoT@P males increased AGI latencies during the third test with an estrous female. B. T@P males decreased mount latencies across the three behavior tests with an estrous female, whereas noT@P males showed no change in mount latency across the three behavior tests. These data suggest that pubertal gonadal hormones have lasting, facilitatory effects on adult male motivation to engage in sexual behavior with a female. (Unpublished data from animal subjects in Schulz, K. M., Richardson, H. N., Zehr, J. L., Osetek, A. J., Menard, T. A., and Sisk, C. L., 2004).
Prepubertal behavioral responses to steroids
One of the enduring puzzles of adolescent behavioral development is why activation of reproductive behavior in response to steroid exposure is attenuated in prepubertal male hamsters. If low levels of androgens before puberty limit the expression of male sexual behavior in prepubertal males, then supplementing endogenous androgens in prepubertal males should elicit mating. This turns out not to be the case (Meek et al., 1997; Romeo, Cook-Wiens, Richardson, and Sisk, 2001; Romeo, Wagner, Jansen, Diedrich, and Sisk, 2002b), in spite of the fact that the number and distribution of AR and ER throughout the mating circuit are similar in hormone-treated prepubertal and adult castrates (Meek et al., 1997; Romeo, Diedrich, and Sisk, 1999; Romeo et al., 2002a). Therefore, it appears that androgens and AR are necessary but not sufficient for expression of male sexual behavior.
Efforts to identify factors that limit sexual activity before puberty have thus far been mixed. Fos responses to chemosensory cues from estrous females are similar in prepubertal and adult male hamsters (Romeo, Parfitt, Richardson, and Sisk, 1998). These data demonstrate that sensory transduction mechanisms are mature before puberty. Thus, juvenile males are able to detect chemosensory cues from females; where they differ from adults is in how they respond to those cues. One potential explanation is that prepubertal males are not motivated to engage in sexual behavior. We have found that prepubertal male hamsters do not display increased dopaminergic responses in the MPOA in response to female pheromones, whereas sexually-naïve adult males display robust MPOA dopaminergic responses to the same stimuli (Fig. 5, Schulz, Richardson, Romeo, Morris, Lookingland, and Sisk, 2003). Similarly, prepubertal males fail to show the adult-typical increase in circulating testosterone following exposure to female pheromones (Parfitt, Thompson, Richardson, Romeo, and Sisk, 1999). Thus, female pheromones appear to be an unconditioned stimulus for neurochemical and neuroendocrine responses in adult, but not prepubertal males, suggesting that the salience of these socially relevant sensory stimuli changes over pubertal development, possibly related to the acquisition of rewarding properties and sexual motivation. In addition, although testosterone does facilitate AGI of a female in prepubertal males, this effect depends on whether or not the male has had previous exposure to an estrous female. Perhaps surprisingly, testosterone-treatment decreases the latency and increases the duration of AGI only in sexually-naïve prepubertal males (Fig. 6). Furthermore, prepubertal males that have had one previous experience with a female display much longer AGI latencies and shorter AGI durations than males interacting with receptive females for the first time (Fig. 6). These data suggest that interactions with an estrous female are aversive rather than rewarding prior to puberty, thereby eliminating any facilitating effects of testosterone on AGI during subsequent interactions with a female. It would be interesting to know whether the negative behavioral consequences of early exposure to an estrous female persist into adolescence and adulthood, especially given that repeated exposure to estrous females during adolescence generally facilitates the expression of male reproductive behavior (Molenda-Figueira, Salas-Ramirez, Schulz, Zehr, Montalto, and Sisk, 2007).

Fig. 5
Prepubertal and adult male medial preoptic area (MPOA) dopaminergic responses to female pheromones contained in vaginal secretions. Adult males show increases in MPOA dopaminergic activity with exposure to female vaginal secretions, whereas prepubertal males do not display increased MPOA dopaminergic responses to female pheromones. (Redrawn from Schulz, K. M., Richardson, H. N., Romeo, R. D., Morris, J. A., Lookingland, K. J., and Sisk, C. L., 2003).

Although prepubertal androgen treatment cannot induce copulation, recent work from our laboratory suggests that the hamster nervous system is sensitive to the organizing actions of testosterone on reproductive behavior prior to adolescence (Schulz, Zehr, Salas-Ramirez, and Sisk, 2007). Castration plus 19 days of testosterone exposure before or during but not after adolescence facilitated mounting behavior when testosterone was replaced in adulthood. Males exposed to testosterone prepubertally also displayed more intromissions in adulthood than males exposed to testosterone during or after puberty (Schulz et al., 2007). These data suggest that the ability of testosterone to organize behavioral neural circuits decreases with age, and that adolescence marks the end of a protracted postnatal sensitive period for exposure to testosterone.
Pharmacologic androgens
The preceding data suggest that endogenous gonadal steroids enhance motivated behaviors during adolescence. Now, what happens if one self-administers androgens at levels up to 100x normal physiologic concentrations? This is the problem of anabolic-androgenic steroid (AAS) abuse (reviewed in Brower, 2002; Clark and Henderson, 2003). A brief digression is appropriate here: all AAS are derivatives of testosterone, all AAS have a carbon skeleton with 4 fused rings, most have 19 carbons. AAS are used principally for their anabolic (muscle-building) effects. However, as their name implies, AAS also have androgenic properties. Testosterone is a logical choice in animal studies for exploring fundamental mechanisms of androgen reward. It remains a popular choice for human users as well, most often in the form of long-acting testosterone esters such as testosterone propionate. In 2006, testosterone was the single most-common banned substance detected in urine tests at WADA-accredited laboratories (WADA, 2006). Testosterone accounted for the largest fraction (34%) of AAS-positive urine tests at the 2000 Sydney Olympic Games (Van Eenoo and Delbeke, 2003). Likewise, in urine tests of AAS users, 41% tested positive for testosterone (Brower, Catlin, Blow, Eliopulos, Beresford, 1991). At high doses, AAS produce significant behavioral changes. In particular, because of their close relationship to testosterone, AAS use in the teen years would appear to perturb the normal steroid milieu of the developing human adolescent nervous system, including the quantity, timing, and type of steroid exposure.
As with other illicit drugs, human AAS abuse is a problem of adolescence. According to the 1994 National Household Survey on Drug Use (SAMHSA/OAS, 1996), steroid use peaks in late adolescence at 18 years of age. Moreover, in the Monitoring the Future survey (Johnston, O’Malley and Bachman, 2003), the lifetime incidence of steroid use among high school seniors (2.7%) was comparable to that for crack cocaine (3.5%) or heroin (1.4%). Steroid use is also increasingly common at younger ages: 2.5% of 8th grade students (13–14 years) have used steroids, similar to the incidence of crack (2.5%) and heroin use (1.6%). This trend toward AAS use in early teens is particularly troubling in view of concerns 1) that adolescents may be particularly vulnerable to abuse AAS, and 2) that adolescent exposure to AAS at pharmacologic levels has the potential to substantially alter the normal maturation of brain and behavior to produce exaggerated morphological and behavioral responses, acutely and chronically.
Inappropriate aggression is the behavioral response most often associated with human AAS abuse. In published case reports, steroid use has been implicated in several violent murders (Conacher and Workman, 1989; Pope and Katz, 1990; Pope, Kouri, Powell, Campbell, and Katz, 1996; Schulte, Hall, and Boyer, 1993). In surveys of current AAS users, elevated aggression and irritability were the most common behavioral side effects of AAS use (Bond, Choi, and Pope, 1995; Galligani, Renck, and Hansen, 1996; Midgley, Heather, and Davies, 2001; Parrott, Choi, and Davies, 1994; Perry, Kutscher, Lund, Yates, Holman, and Demers, 2003). However, given the range of androgen exposures, the variety of psychiatric symptoms, and the potential for pre-existing psychiatric dysfunction, it is difficult to determine the precise role of AAS in these cases of human aggression. Results from prospective studies of human volunteers receiving injections of AAS have been mixed: Tricker et al (1996) and O’Connor et al (2004) reported no increases in angry behavior while other studies have observed increased aggression (Daly, Su, Schmidt, Pickar, Murphy, and Rubinow, 2001; Hannan, Friedl, Zold, Kettler, and Plymate, 1991; Kouri, Lukas, Pope, and Oliva, 1995; Pope and Katz, 1994; Su, Pagliaro, Schmidt, Pickar, Wolkowitz, and Rubinow, 1993). Nonetheless, it is important to keep in mind that the doses administered to human volunteers are much lower than the doses advocated on body building websites, and the duration of treatment is generally short. Thus, on balance, it seems to fair to conclude that AAS have the potential to enhance agonistic behavior, at least in susceptible individuals. Pope et al (1994) found that AAS elicit psychiatric symptoms in vulnerable individuals.
Animal studies have also provided compelling evidence for AAS-induced aggression. Adolescent male hamsters treated chronically with high-dose steroids have shorter attack latencies and a greater number of attacks and bites towards a male intruder compared with untreated males (Harrison, Connor, Nowak, Nash, and Melloni, 2000; Melloni, Connor, Hang, Harrison, and Ferris, 1997). Similarly, a mild provocation (tail-pinch) produces a persistent increase in aggression in adolescent male rats, including aggression towards females (Cunningham and McGinnis, 2006). Of even greater concern, adolescent exposure to AAS in hamsters causes lasting increases in agonistic behavior that persist after steroid use is discontinued (Grimes and Melloni, 2006). These behavioral changes are accompanied by lasting remodeling of neural circuitry in the anterior hypothalamus. In particular, adolescent AAS exposure in hamsters enhances arginine vasopressin (AVP, Grimes and Melloni, 2006) and downregulates serotonin and the serotonergic 5HT1A and 5HT1B receptors (Ricci, Rasakham, Grimes, and Melloni, 2006). It should come as no surprise that AAS alter brain levels of AR as well. Chronic exposure to either testosterone or nandrolone upregulates cell nuclear AR in male rats (Menard and Harlan, 1993; Wesson and McGinnis, 2006). Thus, there is the potential for AAS to enhance androgen-dependent behaviors both by supplementing endogenous androgens and by increasing androgenic responsiveness via increased AR expression.
Compared with agonistic behavior, AAS have a less marked effect on mating behavior in male rodents, and the response depends on the particular steroid used (reviewed in Clark and Henderson, 2003). In male hamsters consuming testosterone in oral solutions, ejaculations increased in a dose-dependent manner (Wood, 2002). However, neither testosterone nor nandrolone enhanced mating in adolescent male rats. Stanozolol, a relatively less potent AAS with minimal androgenic activity, actually inhibited both mating and aggression (Farrell and McGinnis, 2003), presumably by reducing endogenous androgen levels.
It is particularly important to note that adolescent and adult hamsters can show different behavioral responses to AAS exposure. While AAS markedly enhanced agonistic behavior in adolescent males, the same treatment in adulthood produced only a modest increase in aggressive behavior and significantly decreased sexual behavior (Salas-Ramirez, Montaldo and Sisk, 2008). This is consistent with the concept of adolescence as a sensitive period for androgen action. Furthermore, just as adult male hamsters acquire tolerance to exogenous testosterone (Peters and Wood, 2005), we believe that developing males acquire tolerance to testosterone as they mature. Thus, the effects of AAS change across adolescent development, and adolescent AAS exposure can cause excessive aggressive and sexual behavior patterns that may persist into adulthood.
Reinforcing effects of androgens
Mating and fighting are each rewarding (at least if you win the fight). Male rats will press a lever repeatedly in order to copulate with a female (Everitt and Stacey, 1987). Similarly, male mice and female hamsters will form a conditioned place preference (CPP) for locations where they have previously won fights (Martinez, Guillen-Salazar, Salvador, and Simon, 1995; Meisel and Joppa, 1994). If AAS can enhance rewarding social behaviors above levels normally observed in gonad-intact males, it is logical to expect that testosterone itself might be rewarding. This has been tested using two well-established animal models for reward and reinforcement: CPP and self-administration. The results of these studies demonstrate that testosterone is reinforcing in an experimental context where anabolic effects and athletic performance are irrelevant. With CPP, the test substance is repeatedly paired with a unique environment (for example, a particular chamber in the testing apparatus). Once the animal associates the reinforcing test substance with that environment, he will seek out the environment even in the absence of reward. The first reports of androgen reward in laboratory animals used systemic injections of testosterone to induce CPP in male mice (Arnedo, Salvador, Martinez-Sanchis, and Gonzalez-Bono, 2000; Arnedo, Salvador, Martinez-Sanchis, and Pellicer, 2002) and rats (Alexander, Packard, and Hines, 1994; de Beun, Jansen, Slangen, and Van de Poll, 1992). Subsequently, our laboratory used self-administration of testosterone to demonstrate androgen reinforcement (Johnson and Wood, 2001). We found that male hamsters will voluntarily consume oral solutions of testosterone using both 2-bottle choice tests and food-induced drinking. In later studies, we demonstrated iv self-administration in male rats and hamsters (Wood, Johnson, Chu, Schad, and Self, 2004). Intravenous delivery eliminates potential confounding effects of taste or gut fill on androgen intake.
In the context of AAS abuse, it is important to differentiate between central and peripheral effects of androgens. Since testosterone has widespread effects throughout the body, it could be argued that reward and reinforcement with systemic testosterone injections is secondary to testosterone’s systemic anabolic and androgenic actions. In other words, maybe testosterone reduces muscle fatigue and improves joint function so that animals just feel better. Indeed, this explanation has been used in the clinical literature (albeit without experimental evidence) to argue against the potential for dependence and addiction to AAS (DiPasquale, 1998). However, Packard et al (Packard, Cornell, and Alexander, 1997) showed that injections of testosterone directly into the rat brain can induce CPP. Likewise, our laboratory has demonstrated intracerebroventricular (icv) testosterone self-administration in male hamsters (Wood et al., 2004). Intracerebral CPP and icv self-administration with testosterone argue for central targets mediating androgen reinforcement.
It is notable that testosterone reinforcement does not necessarily follow the same mechanisms previously established for steroid effects on sexual behavior. As discussed previously, the MPOA is a key site for organization of male rodent sexual behavior (Hull, Meisel, and Sachs, 2002). In hamsters, the MPOA has abundant steroid receptors, and testosterone implants in MPOA restore sexual activity in long-term castrates (Wood and Swann, 1999). The time-course of these steroid effects is slow: mounting behavior persists for weeks after orchidectomy, and extended steroid exposure is necessary to restore mating in long-term castrates (Noble and Alsum, 1975). However, injections of testosterone into MPOA of male rats fail to induce CPP (King, Packard, and Alexander, 1999). This suggests that other brain regions are important for androgen reinforcement.
In contrast, male rats will form a CPP to testosterone injections in Acb (Packard et al., 1997). As with other drugs of abuse, DA is likely to be a key neurotransmitter for testosterone reinforcement: CPP induced by systemic testosterone injection is blocked by D1 and D2 dopamine receptor antagonists (Schroeder and Packard, 2000). However, unlike other drugs of abuse, our studies in hamsters suggest that testosterone does not induce Acb DA release (Triemstra, Sato, and Wood, in press). Likewise, studies of male rats show that androgens have no effect on basal DA levels or amphetamine-stimulated DA release (Birgner, Kindlundh-Hogberg, Nyberg, and Bergstrom, 2006; but also see Clark, Lindenfeld, and Gibbons, 1996). Furthermore, testosterone exerts a relatively minor influence on Acb DA tissue levels (Thiblin, Finn, Ross, and Stenfors, 1999). Together, these data suggest that although testosterone reinforcement may ultimately alter DA activity in Acb, the mechanisms may be distinct from those of cocaine or other stimulants. In this regard, recent data suggest that chronic exposure to AAS may alter sensitivity to DA by altering DA metabolism (Kurling, Kankaanpaa, Ellermaa, Karila, and Seppala, 2005), levels of DA receptors (Kindlundh, Lindblom, Bergstrom, Wikberg, and Nyberg, 2001; Kindlundh, Lindblom, and Nyberg, 2003) or the DA transporter (Kindlundh, Bergstrom, Monazzam, Hallberg, Blomqvist, Langstrom, and Nyberg, 2002).
At the present time, the specific steroid signals, receptors and brain sites of action for testosterone reinforcement are unknown. Based on a recent study of hamsters from our laboratory, the reinforcing effects of testosterone appear to be mediated by both androgens and estrogens (DiMeo and Wood, 2006). Commonly-abused AAS include both aromatizable and non-aromatizable androgens (Gallaway, 1997; WADA, 2006). This implies that both AR and ER may transduce steroidal stimuli for reward. There is the additional possibility that testosterone reinforcement may be mediated by a combination of classical and non-genomic receptors.
Several lines of evidence point to the actions of non-genomic receptors in the reinforcing effects of AAS. In addition to the sparse distribution of AR in Acb and VTA, the time-course of androgen reinforcement is rapid (<30 min), and signal processing through classical AR may not be fast enough for reinforcement. Accordingly, to test the role of non-genomic AR in AAS reinforcement, we utilized two complementary techniques (Fig. 7). In one experiment (Sato, Johansen, Jordan, and Wood, 2006), we allowed rats with the testicular feminization mutation (Tfm, see this issue) to self-administer dihydrotestosterone (DHT), a non-aromatizable androgen. The Tfm mutation greatly diminishes ligand binding at AR. Nonetheless, Tfm rats and their wild-type male siblings self-administered roughly the same amount of DHT. This argues for non-genomic effects of DHT. In a subsequent study, we determined if male hamsters would self-administer DHT conjugated to bovine serum albumin (BSA, Fig. 8, Sato and Wood, 2007). DHT-BSA conjugates are membrane-impermeable; thus their effects are limited to the cell surface. Hamsters self-administered DHT, as previously demonstrated (DiMeo and Wood, 2006). They showed a similar preference for DHT-BSA conjugates, but failed to self-administer BSA alone.
These data point toward a central role for cell surface ARs in androgen reinforcement. Currently, the exact nature of such receptors is not known. It has been suggested that androgens may act at the cell surface through binding to dedicated membrane AR (Thomas, Dressing, Pang, Berg, Tubbs, Benninghoff, and Doughty, 2006, also see this issue). This may be in the form of extra-nuclear classical AR as reported in hippocampus (Sarkey et al., in this issue). Alternatively, previous studies have also described steroid-binding sites on other neurotransmitter systems. Specifically, a variety of steroid hormones including AAS can allosterically modulate the GABA-A receptor (Henderson, 2007; Lambert, Belelli, Peden, Vardy, and Peters, 2003). Likewise, sulfated neurosteroids can modify activity of N-methyl-D-aspartate receptor subtypes (Malayev, Gibbs, and Farb, 2002) receptors. This is an important area for future research.
Why should there be a membrane AR? As discussed previously, there is a close association between androgen secretion and rewarding social behaviors. We can speculate that the increase in testosterone secretion that follows mating or fighting serves to reinforce the behavior. If so, it is necessary to have a rapid coupling of stimulus (behavior) and reward (testosterone). This can best be achieved through binding to membrane AR. In this regard, it would be of interest to determine if clamping androgen secretion during mating reduces the rewarding effects of sexual behavior.
Summary
Here we review the evidence that androgens are potent mediators of adult motivated behaviors, and further, that the timing of androgen exposure during development programs androgen-dependent motivated behavior in adulthood. Anabolic steroids are fast becoming a favored drug of abuse by adolescents in the US. While AAS may not have the addictive potency of cocaine or heroin, we are just beginning to understand the potential for androgen reinforcement and addiction. In particular, as youth sports become more competitive, there is increasing pressure on developing athletes to use steroids, starting at younger ages. This trend is troubling in view of new evidence for steroid-sensitive neural maturation in adolescents.
Despite increased awareness by both the public and scientific communities of the profound neural changes accompanying adolescence, experimental study of the developmental neurobiology of puberty has been limited. Animal models of adolescent development are needed to investigate how the timing of hormone exposure during development increases an individual’s risk for psychopathology and drug use, and what types of experiences mitigate or amplify the behavioral effects of deviations in pubertal timing. For example, social factors such as peer influence exacerbate the effects of pubertal timing for substance and alcohol use (Biehl, Natsuaki, and Ge, 2007; Patton, Novy, Lee, and Hickok, 2004; Simons-Morton and Haynie, 2003; Wichstrom and Pedersen, 2001). Animal models of pubertal timing will also inform human research efforts, and potentially lead to more effective therapeutic interventions during adolescence.
Acknowledgments
We thank Eleni Antzoulatos, Cortney Ballard, Lucy Chu, Kelly Peters, Jennifer Triemstra, Jane Venier, Lisa Rogers, and Pamela Montalto for assistance with these studies. This work supported by grants from the NIH (DA12843 to RIW, MH68764 to CLS, and MH070125 to KMS).
Footnotes
Publisher’s Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Packard MG, Hines M. Testosterone has rewarding affective properties in male rats: implications for the biological basis of sexual motivation. Behavioral Neuroscience. 1994;108:424–8. [PubMed]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews. 2003;27:3–18. [PubMed]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–8. [PubMed]
- Arnedo MT, Salvador A, Martinez-Sanchis S, Gonzalez-Bono E. Rewarding properties of testosterone in intact male mice: a pilot study. Pharmacology, Biochemistry & Behavior. 2000;65:327–32.
- Arnedo MT, Salvador A, Martinez-Sanchis S, Pellicer O. Similar rewarding effects of testosterone in mice rated as short and long attack latency individuals. Addiction Biology. 2002;7:373–9. [PubMed]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Hormones & Behavior. 1985;19:469–98. [PubMed]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cerebral Cortex. 2000;10:1014–27. [PubMed]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–84. [PubMed]
- Biehl MC, Natsuaki MN, Ge XJ. The influence of pubertal timing on alcohol use and heavy drinking trajectories. Journal of Youth and Adolescence. 2007;36:153–167.
- Birgner C, Kindlundh-Hogberg AM, Nyberg F, Bergstrom L. Neuroscience Letters. 2006. Altered extracellular levels of DOPAC and HVA in the rat nucleus accumbens shell in response to sub-chronic nandrolone administration and a subsequent amphetamine challenge.
- Bond AJ, Choi PY, Pope HG., Jr Assessment of attentional bias and mood in users and non-users of anabolic-androgenic steroids. Drug & Alcohol Dependence. 1995;37:241–5. [PubMed]
- Brower KJ. Anabolic steroid abuse and dependence. Current Psychiatry Reports. 2002;4:377–87. [PubMed]
- Brower KJ, Catlin DH, Blow FC, Eliopulos GA, Beresford TP. Clinical asessment and urine testing for anabolic-androgenic steroid abuse and dependence. American Journal of Drug & Alcohol Abuse. 1991;17:161–171. [PubMed]
- Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Research Bulletin. 1997;44:365–75. [PubMed]
- Chung WC, De Vries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. Journal of Neuroscience. 2002;22:1027–33. [PubMed]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neuroscience & Biobehavioral Reviews. 2003;27:413–36. [PubMed]
- Clark AS, Lindenfeld RC, Gibbons CH. Anabolic-androgenic steroids and brain reward. Pharmacology, Biochemistry & Behavior. 1996;53:741–5.
- Conacher GN, Workman DG. Violent crime possibly associated with anabolic steroid use. American Journal of Psychiatry. 1989;146:679. [PubMed]
- Cunningham RL, McGinnis MY. Physical provocation of pubertal anabolic androgenic steroid exposed male rats elicits aggression towards females. Hormones & Behavior. 2006;50:410–6. [PubMed]
- Daly RC, Su TP, Schmidt PJ, Pickar D, Murphy DL, Rubinow DR. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Archives of General Psychiatry. 2001;58:172–7. [PubMed]
- Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–8. [PubMed]
- de Beun R, Jansen E, Slangen JL, Van de Poll NE. Testosterone as appetitive and discriminative stimulus in rats: sex- and dose-dependent effects. Physiology & Behavior. 1992;52:629–34. [PubMed]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Annals of Neurology. 1978;4:345–56. [PubMed]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–8. [PMC free article] [PubMed]
- DiMeo AN, Wood RI. Self-administration of estrogen and dihydrotestosterone in male hamsters. Hormones & Behavior. 2006;49:519–26. [PubMed]
- DiPasquale M. Anabolic Steroids. In: Tarter RE, Ammerman RT, Ott PJ, editors. Handbook of Substance Abuse. Plenum Press; NY: 1998. pp. 547–565.
- Dominguez J, Riolo JV, Xu Z, Hull EM. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. Journal of Neuroscience. 2001;21:349–355. [PubMed]
- Dominguez JM, Hull EM. Stimulation of the medial amygdala enhances medial preoptic dopamine release: implications for male rat sexual behavior. Brain Research. 2001;917:225–229. [PubMed]
- Du J, Lorrain DS, Hull EM. Castration decreases extracellular, but increases intracellular, dopamine in medial preoptic area of male rats. Brain Research. 1998;782:11–17. [PubMed]
- Eckenhoff MF, Rakic P. Nature and fate of proliferative cells in the hippocampal dentate gyrus during the life span of the rhesus monkey. Journal of Neuroscience. 1988;8:2729–47. [PubMed]
- Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): II. Effects of preoptic area lesions, castration, and testosterone. Journal of Comparative Psychology. 1987;101:407–19. [PubMed]
- Gallaway S. The Steroid Bible. Belle International Press; Sacramento, CA: 1997.
- Galligani N, Renck A, Hansen S. Personality profile of men using anabolic androgenic steroids. Hormones & Behavior. 1996;30:170–5. [PubMed]
- Grimes JM, Melloni RH., Jr Prolonged alterations in the serotonin neural system following the cessation of adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2006;120:1242–51. [PubMed]
- Hannan CJ, Jr, Friedl KE, Zold A, Kettler TM, Plymate SR. Psychological and serum homovanillic acid changes in men administered androgenic steroids. Psychoneuroendocrinology. 1991;16:335–43. [PubMed]
- Harrison RJ, Connor DF, Nowak C, Nash K, Melloni RH., Jr Chronic anabolic-androgenic steroid treatment during adolescence increases anterior hypothalamic vasopressin and aggression in intact hamsters. Psychoneuroendocrinology. 2000;25:317–38. [PubMed]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacology, Biochemistry & Behavior. 2007;86:327–33.
- Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52:1439–53. [PMC free article] [PubMed]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. Journal of Neuroscience. 1995;15:7465–7471. [PubMed]
- Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. Academic Press; New York: 2002. pp. 3–137.
- Hull EM, Wood RI, McKenna KE. Neurobiology of male sexual behavior. In: Neill JD, editor. Physiology of Reproduction. Vol. 1. Elsevier Press; New York: 2006. pp. 1729–1824.
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–78. [PubMed]
- Johnson LR, Wood RI. Oral testosterone self-administration in male hamsters. Neuroendocrinology. 2001;73:285–92. [PubMed]
- Johnston LD, O’Malley PM, Bachman JG. Secondary school students (NIH Publication No. 03–5375) I. Bethesda, MD: National Institute on Drug Abuse; 2003. Monitoring the Future national survey results on drug use, 1975–2002.
- Kaiser Family Foundation. US teen sexual activity. 2005. pp. #3040–02.
- Kashon ML, Hayes MJ, Shek PP, Sisk CL. Regulation of brain androgen receptor immunoreactivity by androgen in prepubertal male ferrets. Biology of Reproduction. 1995;52:1198–205. [PubMed]
- Kindlundh AM, Bergstrom M, Monazzam A, Hallberg M, Blomqvist G, Langstrom B, Nyberg F. Dopaminergic effects after chronic treatment with nandrolone visualized in rat brain by positron emission tomography. Progress in NeuroPsychopharmacology & Biological Psychiatry. 2002;26:1303–8.
- Kindlundh AM, Lindblom J, Bergstrom L, Wikberg JE, Nyberg F. The anabolic-androgenic steroid nandrolone decanoate affects the density of dopamine receptors in the male rat brain. European Journal of Neuroscience. 2001;13:291–6. [PubMed]
- Kindlundh AM, Lindblom J, Nyberg F. Chronic administration with nandrolone decanoate induces alterations in the gene-transcript content of dopamine D(1)- and D(2)-receptors in the rat brain. Brain Research. 2003;979:37–42. [PubMed]
- King BE, Packard MG, Alexander GM. Affective properties of intra-medial preoptic area injections of testosterone in male rats. Neuroscience Letters. 1999;269:149–52. [PubMed]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. Journal of Neuropsychiatry & Clinical Neurosciences. 1997;9:482–97. [PubMed]
- Kouri EM, Lukas SE, Pope HG, Jr, Oliva PS. Increased aggressive responding in male volunteers following the administration of gradually increasing doses of testosterone cypionate. Drug & Alcohol Dependence. 1995;40:73–9. [PubMed]
- Kritzer MF. Long-term gonadectomy affects the density of tyrosine hydroxylase- but not dopamine-beta-hydroxylase-, choline acetyltransferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cerebral Cortex. 2003;13:282–296. [PubMed]
- Kurling S, Kankaanpaa A, Ellermaa S, Karila T, Seppala T. The effect of sub-chronic nandrolone decanoate treatment on dopaminergic and serotonergic neuronal systems in the brains of rats. Brain Research. 2005;1044:67–75. [PubMed]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Progress in Neurobiology. 2003;71:67–80. [PubMed]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Review. 2006;30:718–29.
- Lesting J, Neddens J, Teuchert-Noodt G. Ontogeny of the dopamine innervation in the nucleus accumbens of gerbils. Brain Research. 2005;1066:16–23. [PubMed]
- Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. British Journal of Pharmacology. 2002;135:901–9. [PMC free article] [PubMed]
- Martinez M, Guillen-Salazar F, Salvador A, Simon VM. Successful intermale aggression and conditioned place preference in mice. Physiology & Behavior. 1995;58:323–8. [PubMed]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Hormones & Behaviors. 1997;31:75–88.
- Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiology & Behavior. 1994;56:1115–8. [PubMed]
- Melloni RH, Jr, Connor DF, Hang PT, Harrison RJ, Ferris CF. Anabolic-androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiology and Behavior. 1997;61:359–64. [PubMed]
- Menard C, Harlan R. Up-regulation of androgen receptor immunoreactivity in the rat brain by androgenic-anabolic steroids. Brain Research. 1993;622:226–236. [PubMed]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. Journal of Neuroscience. 1996;16:595–604. [PubMed]
- Midgley SJ, Heather N, Davies JB. Levels of aggression among a group of anabolic-androgenic steroid users. Medicine, Science & the Law. 2001;41:309–14.
- Molenda-Figueira HN, Salas-Ramirez KY, Schulz KM, Zehr JL, Montalto PR, Sisk CL. Adolescent social experience restores adult ejaculatory behavior in male Syrian hamsters lacking pubertal testosterone. Society for Behavioral Neuroendocrinology; Pacific Grove, CA: 2007.
- Noble RG, Alsum PB. Hormone dependent sex dimorphisms in the golden hamster (Mesocricetus auratus) Physiology & Behavior. 1975;14:567–74. [PubMed]
- Nunez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. Journal of Comparative Neurology. 2001;436:32–41. [PubMed]
- Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. Journal of Neurobiology. 2002;52:312–21. [PubMed]
- O’Connor DB, Archer J, Wu FC. Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over study. Journal of Clinical Endocrinology & Metabolism. 2004;89:2837–45. [PubMed]
- Packard MG, Cornell AH, Alexander GM. Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behavioral Neuroscience. 1997;111:219–24. [PubMed]
- Parfitt DB, Thompson RC, Richardson HN, Romeo RD, Sisk CL. GnRH mRNA increases with puberty in the male Syrian hamster brain. Journal of Neuroendocrinology. 1999;11:621–7. [PubMed]
- Parrott AC, Choi PY, Davies M. Anabolic steroid use by amateur athletes: effects upon psychological mood states. Journal of Sports Medicine & Physical Fitness. 1994;34:292–8. [PubMed]
- Patton PE, Novy MJ, Lee DM, Hickok LR. The diagnosis and reproductive outcome after surgical treatment of the complete septate uterus, duplicated cervix and vaginal septum. American Journal of Obstetrics & Gynecology. 2004;190:1669–75. 1675–8. [PubMed]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54:255–66. [PubMed]
- Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. Journal of Forensic Sciences. 2003;48:646–51. [PubMed]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130:971–81. [PubMed]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Research. 1990;530:345–348. [PubMed]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. [PubMed]
- Pinos H, Collado P, Rodriguez-Zafra M, Rodriguez C, Segovia S, Guillamon A. The development of sex differences in the locus coeruleus of the rat. Brain Research Bulletin. 2001;56:73–8. [PubMed]
- Pope HG, Jr, Katz DL. Homicide and near-homicide by anabolic steroid users.[see comment] Journal of Clinical Psychiatry. 1990;51:28–31. [PubMed]
- Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Archives of General Psychiatry. 1994;51:375–82. [PubMed]
- Pope HG, Jr, Kouri EM, Powell KF, Campbell C, Katz DL. Anabolic-androgenic steroid use among 133 prisoners. Comprehensive Psychiatry. 1996;37:322–7. [PubMed]
- Putnam SK, Du J, Sato S, Hull EM. Testosterone restoration of copulatory behavior correlates with medial preoptic dopamine release in castrated male rats. Hormones & Behavior. 2001;39:216–224. [PubMed]
- Putnam SK, Sato S, Hull EM. Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Hormones & Behavior. 2003;44:419–26. [PubMed]
- Putnam SK, Sato S, Riolo JV, Hull EM. Effects of testosterone metabolites on copulation, medial preoptic dopamine, and NOS-immunoreactivity in castrated male rats. Hormones & Behavior. 2005;47:513–522. [PubMed]
- Rankin SL, Partlow GD, McCurdy RD, Giles ED, Fisher KR. Postnatal neurogenesis in the vasopressin and oxytocin-containing nucleus of the pig hypothalamus. Brain Research. 2003;971:189–96. [PubMed]
- Ricci LA, Rasakham K, Grimes JM, Melloni RH., Jr Serotonin-1A receptor activity and expression modulate adolescent anabolic/androgenic steroid-induced aggression in hamsters. Pharmacology, Biochemistry & Behavior. 2006;85:1–11.
- Romeo RD, Cook-Wiens E, Richardson HN, Sisk CL. Dihydrotestosterone activates sexual behavior in adult male hamsters but not in juveniles. Physiology & Behavior. 2001;73:579–84. [PubMed]
- Romeo RD, Diedrich SL, Sisk CL. Estrogen receptor immunoreactivity in prepubertal and adult male Syrian hamsters. Neuroscience Letters. 1999;265:167–70. [PubMed]
- Romeo RD, Parfitt DB, Richardson HN, Sisk CL. Pheromones elicit equivalent levels of Fos-immunoreactivity in prepubertal and adult male Syrian hamsters. Hormones & Behavior. 1998;34:48–55. [PubMed]
- Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neuroscience & Biobehavioral Reviews. 2002a;26:381–91. [PubMed]
- Romeo RD, Wagner CK, Jansen HT, Diedrich SL, Sisk CL. Estradiol induces hypothalamic progesterone receptors but does not activate mating behavior in male hamsters (Mesocricetus auratus) before puberty. Behavioral Neuroscience. 2002b;116:198–205. [PubMed]
- Salas-Ramirez KY, Montalto PR, Sisk CL. Anabolic androgenic steroids (AAS) differentially affect social behaviors in adolescent and adult male Syrian hamsters. Hormones & Behavior. 2008 in press.
- SAMHSA/OAS. DHHS Publication No (SMA) 1996. 1994 National Household Survey on Drug Abuse, Main Findings 1994; pp. 96–3085.
- SAMHSA/OAS. Results from the 2004 National Survey on Drug Use and Health: National Findings. 2005. NSDUH Series H-28, DHHS Publication No. SMA 05–4062.
- Sato SM, Johansen J, Jordan CL, Wood RI. Androgen self-administration in Tfm rats. 10th Annual Meeting of Society for Behavioral Neuroendocrinology.2006.
- Sato SM, Wood RI. Self-administration of membrane-impermeable anabolic-androgenic steroids (aas) in syrian hamsters. 11th Annual Meeting of Society fof Behavioral Neuroendocrinology.2007.
- Schroeder JP, Packard MG. Role of dopamine receptor subtypes in the acquisition of a testosterone conditioned place preference in rats. Neuroscience Letters. 2000;282:17–20. [PubMed]
- Schulte HM, Hall MJ, Boyer M. Domestic violence associated with anabolic steroid abuse. American Journal of Psychiatry. 1993;150:348. [PubMed]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Hormones & Behavior. 2006;50:477–83. [PubMed]
- Schulz KM, Richardson HN, Romeo RD, Morris JA, Lookingland KJ, Sisk CL. Medial preoptic area dopaminergic responses to female pheromones develop during puberty in the male Syrian hamster. Brain Research. 2003;988:139–45. [PubMed]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones & Behavior. 2004;45:242–9. [PubMed]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular & Cellular Endocrinology. 2006:254–255. 120–6.
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Neuroscience Meeting Planner. Sandiego, CA: Society for Neuroscience; 2007. Is adolescence a second sensitive period for the organizing effects of testosterone on adult male reproductive behavior? 2007 Online.
- Scott JP, Stewart JM, De Ghett VJ. Critical periods in the organization of systems. Developmental Psychobiology. 1974;7:489–513. [PubMed]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–25. [PubMed]
- Simons-Morton BG, Haynie DL. Psychosocial predictors of increased smoking stage among sixth graders. American Journal of Health Behavior. 2003;27:592–602. [PubMed]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26:163–74. [PubMed]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–31. [PubMed]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21:8819–29. [PubMed]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Review. 2000;24:417–63.
- Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269:2760–4. [PubMed]
- Thiblin I, Finn A, Ross SB, Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. British Journal of Pharmacology. 1999;126:1301–6. [PMC free article] [PubMed]
- Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, Doughty K. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids. 2006;71:310–6. [PubMed]
- Tricker R, Casaburi R, Storer TW, Clevenger B, Berman N, Shirazi A, Bhasin S. The effects of supraphysiological doses of testosterone on angry behavior in healthy eugonadal men–a clinical research center study. Journal of Clinical Endocrinology & Metabolism. 1996;81:3754–8. [PubMed]
- Triemstra JL, Nagatani S, Wood RI. Chemosensory cues are essential for mating-induced dopamine release in MPOA of male Syrian hamsters. Neuropsychopharmacology. 2005;30:1436–42. [PubMed]
- Triemstra JL, Sato SM, Wood RI. Testosterone and nucleus accumbens dopamine in the male Syrian hamster. Psychoneuroendocrinology (in press)
- Van Eenoo P, Delbeke FT. The prevalence of doping in Flanders in comparison to the prevalence of doping in international sports. International Journal of Sports Medicine. 2003;24:565–570. [PubMed]
- WADA. Adverse analytical findings reported by accredited laboratories. 2006. http://www.wada-ama.org.
- Wesson DW, McGinnis MY. Stacking anabolic androgenic steroids (AAS) during puberty in rats: a neuroendocrine and behavioral assessment. Pharmacology, Biochemistry & Behavior. 2006;83:410–9.
- Wichstrom L, Pedersen W. Use of anabolic-androgenic steroids in adolescence: winning, looking good or being bad? Journal of Studies on Alcohol. 2001;62:5–13. [PubMed]
- Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology. 2004;171:298–305. [PubMed]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist with in individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995;62:487–97. [PubMed]
- Wood RI, Swann JM. Neuronal integration of chemosensory and hormonal signals that control male sexual behavior. In: Wallen K, Schneider JS, editors. Reproduction in Context. MIT Press; Cambridge: 1999. pp. 423–444.
- Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. Journal of Comparative Neurology. 1990;302:437–46. [PubMed]