Background: Steele et al., 2013 and David Ley’s “Your Brain on Porn – It’s NOT Addictive“.
On March 6th, 2013 David Ley and study spokesperson Nicole Prause teamed up to write a Psychology Today blog post about Steele et al., 2013 called “Your Brain on Porn – It’s NOT Addictive“. Its oh-so-catchy title is misleading as it has nothing to do with Your Brain on Porn or the neuroscience presented there. Instead, David Ley’s March, 2013 blog post limits itself to a fictional account of a single flawed EEG study – Steele et al., 2013.
Ley’s blog post appeared 5 months before Steele et al. was formally published. A month later (April 10th) Psychology Today editors unpublished Ley’s blog post due to controversies surrounding its unsubstantiated claims and Prause’s refusal to provided her unpublished study to anyone else. The day Steele et al., and its extensive associated press went public, Ley re-published his blog post. Ley changed the date of his blog post to July 25 2013, eventually closing comments (Update, 2019: David Ley is now being compensated by porn industry giant xHamster to promote its websites and convince users that porn addiction and sex addiction are myths!).
Prause’s carefully orchestrated PR campaign resulted in worldwide media coverage with all the headlines claiming that sex addiction had been debunked(!). In TV interviews and in the UCLA press release Nicole Prause made two wholly unsupported claims about her EEG study:
- Subjects’ brains did not respond like other addicts.
- Hypersexuality (sex addiction) is best understood as “high desire.”
Neither of those findings are actually in Steele et al. 2013. In fact, the study reported the exact opposite of what Nicole Prause and David Ley claimed:
What Steele et al., 2013 actually stated as its “neurological findings”:
“the P300 mean amplitude for the pleasant–sexual condition was more positive than the unpleasant, and pleasant–non-sexual conditions”
Translation: Frequent porn users had greater cue-reactivity (higher EEG readings) to explicit sexual images relative to neutral pictures. This is exactly the same as what occurs when drug addicts are exposed to cues related their addiction.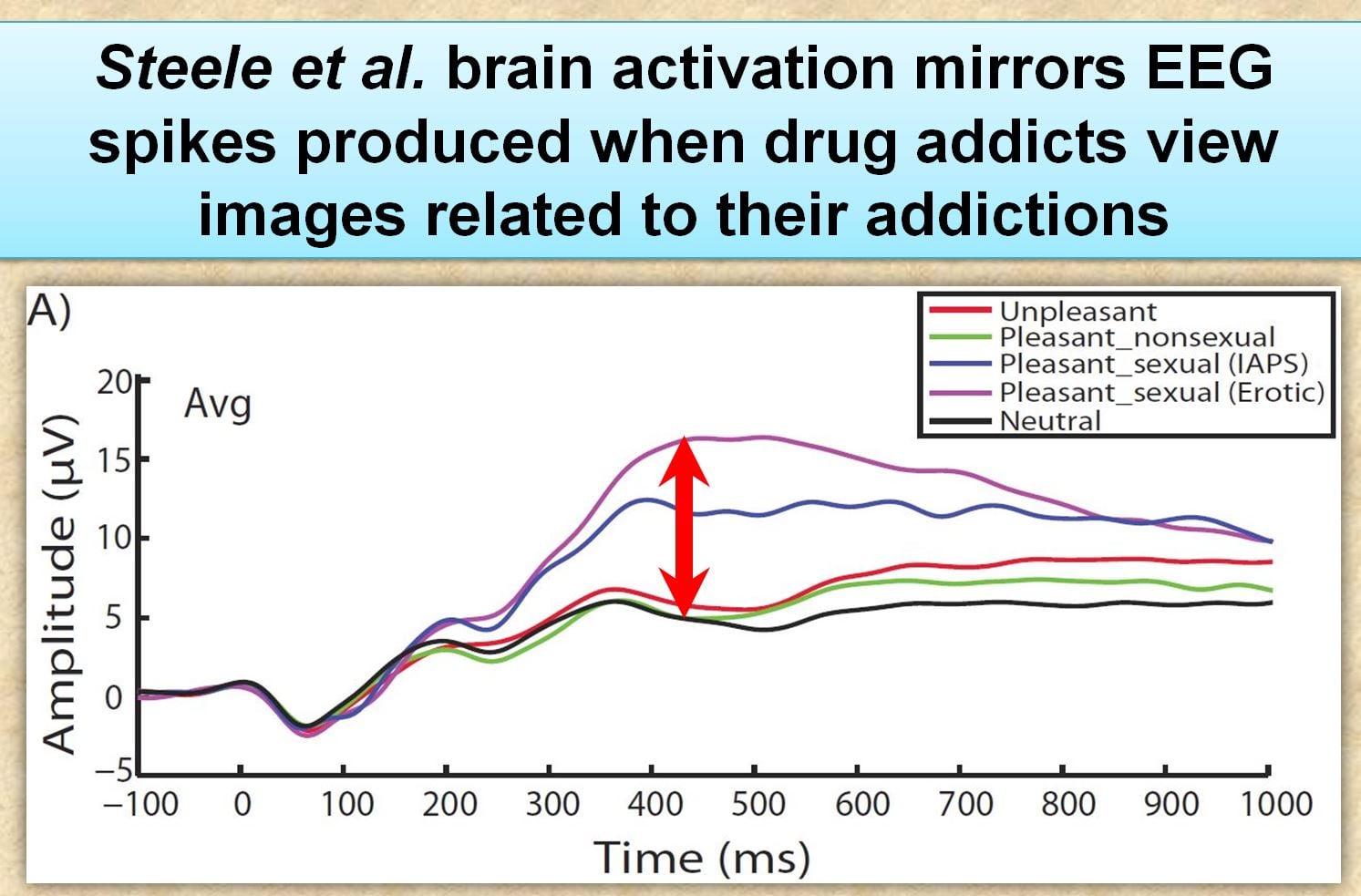
What Steele et al., 2013 actually stated as its “sexual desire” findings:
“Larger P300 amplitude differences to pleasant sexual stimuli, relative to neutral stimuli, was negatively related to measures of sexual desire, but not related to measures of hypersexuality.”
Translation: Negatively means lower desire. Individuals with greater cue-reactivity to porn had lower desire to have sex with a partner (but not lower desire to masturbate). To put another way – individuals with more brain activation and cravings for porn preferred to masturbate to porn than have sex with a real person.
Together these two Steele et al. findings indicate greater brain activity to cues (porn images), yet less reactivity to natural rewards (sex with a person). Both are hallmarks of an addiction, indicating both sensitization and desensitization.
While eight peer-reviewed papers subsequently exposed the truth (below), the first expert to call out Prause for her misrepresentations was senior psychology professor emeritus John A. Johnson {https://www.psychologytoday.com/blog/the-sexual-continuum/201307/new-brain-study-questions-existence-sexual-addiction/comments#comment-556448}. Commenting under the Psychology Today interview of Prause, John A. Johnson revealed the truth:
“My mind still boggles at the Prause claim that her subjects’ brains did not respond to sexual images like drug addicts’ brains respond to their drug, given that she reports higher P300 readings for the sexual images. Just like addicts who show P300 spikes when presented with their drug of choice. How could she draw a conclusion that is the opposite of the actual results? I think it could be due to her preconceptions–what she expected to find.”
John Johnson in yet another comment:
Mustanski asks, “What was the purpose of the study?” And Prause replies, “Our study tested whether people who report such problems [problems with regulating their viewing of online erotica] look like other addicts from their brain responses to sexual images.”
But the study did not compare brain recordings from persons having problems regulating their viewing of online erotica to brain recordings from drug addicts and brain recordings from a non-addict control group, which would have been the obvious way to see if brain responses from the troubled group look more like the brain responses of addicts or non-addicts.
Instead, Prause claims that their within-subject design was a better method, where research subjects serve as their own control group. With this design, they found that the EEG response of their subjects (as a group) to erotic pictures was stronger than their EEG responses to other kinds of pictures. This is shown in the inline waveform graph (although for some reason the graph differs considerably from the actual graph in the published article).
So this group who reports having trouble regulating their viewing of online erotica has a stronger EEG response to erotic pictures than other kinds of pictures. Do addicts show a similarly strong EEG response when presented with their drug of choice? We don’t know. Do normal, non-addicts show a response as strong as the troubled group to erotica? Again, we do not know. We don’t know whether this EEG pattern is more similar to the brain patterns of addicts or non-addicts.
The Prause research team claims to be able to demonstrate whether the elevated EEG response of their subjects to erotica is an addictive brain response or just a high-libido brain response by correlating a set of questionnaire scores with individual differences in EEG response. But explaining differences in EEG response is a different question from exploring whether the overall group’s response looks addictive or not.
Aside from the many unsupported claims in the press, it’s disturbing that Steele et al. passed peer-review, as it suffered from serious methodological flaws: 1) subjects were heterogeneous (males, females, non-heterosexuals); 2) subjects were not screened for mental disorders or addictions; 3) study had no control group for comparison; 4) questionnaires were not validated for porn use or porn addiction (Also see this extensive YBOP critique for a complete dismantling of the claims surrounding Steele et al., 2013).
Before we get to the eight peer-reviewed analyses of Steele et al., 2013 I provide the state of the research in 2020:
- Porn/sex addiction? This page lists 55 neuroscience-based studies (MRI, fMRI, EEG, neuropsychological, hormonal). All provide strong support for the addiction model as their findings mirror the neurological findings reported in substance addiction studies.
- The real experts’ opinions on porn/sex addiction? This list contains 33 recent neuroscience-based literature reviews & commentaries by some of the top neuroscientists in the world. All support the addiction model.
- Signs of addiction and escalation to more extreme material? Over 60 studies reporting findings consistent with escalation of porn use (tolerance), habituation to porn, and even withdrawal symptoms (all signs and symptoms associated with addiction).
- An official diagnosis? The world’s most widely used medical diagnostic manual, The International Classification of Diseases (ICD-11), contains a new diagnosis suitable for porn addiction: “Compulsive Sexual Behavior Disorder.”
- Debunking the Prause and Ley unsupported talking point that “high sexual desire” explains away porn or sex addiction: Over 25 studies falsify the claim that sex & porn addicts “just have high sexual desire”
Eight peer-reviewed analyses of Steele et al., 2013
Over the intervening years many more neuroscience-based studies have been published (MRI, fMRI, EEG, neuropsychological, hormonal). All provide strong support for the addiction model as their findings mirror the neurological findings reported in substance addiction studies. The real experts’ opinions on porn/sex addiction can be seen in this list of 30 recent literature reviews & commentaries (all support the addiction model).
Seven of the peer-reviewed papers chose to analyze what Steele et al. 2013 actually reported – not what Prause put forth in her PR campaign. All describe how the Steele et al. findings lend support to the porn addiction model. The papers are in alignment with the YBOP critique. Three of the papers also describe the study’s flawed methodology and unsubstantiated conclusions. Paper #1 is solely devoted to Steele et al., 2013. Papers 2-8 contain sections analyzing Steele et al., 2013. They are listed by date of publication:
1) ‘High Desire’, or ‘Merely’ An Addiction? A Response to Steele et al. by Donald L. Hilton, Jr., MD. (2014)
The validity of an argument depends on the soundness of its premises. In the recent paper by Steele et al., conclusions are based on the initial construction of definitions relating to ‘desire’ and ‘addiction’. These definitions are based on a series of assumptions and qualifications, the limitations of which are acknowledged by the authors initially, but inexplicably ignored in reaching the firm conclusions the authors make. Yet, the firmness of these conclusions is unwarranted, not only as a result of conceptually problematic initial premises but also due to problematic methodology.
Consider, for instance, the concept of ‘sexual desire’. The first paragraph acknowledges that ‘sexual desires must be consistently regulated to manage sexual behaviors’, and must be controlled when either illegal (pedophilia) or inappropriate (infidelity). The paragraph ends with the inference that the term ‘sexual addiction’ does not describe a problematic entity per se, but that it merely describes a subset of individuals with high levels of desire.
The next paragraph references a paper by Winters et al., which suggests that ‘dysregulated sexuality … may simply be a marker of high sexual desire and the distress associated with managing a high degree of sexual thoughts, feelings, and needs’ (Winters, Christoff, & Gorzalka, 2010). It is based on these assumptions that Steele et al. then proceeds to question a disease model for this ‘distress’ associated with controlling sexual ‘desire’. For a comparison of different ‘desire’ templates, television viewing in children is used as an example. The last two sentences in this paragraph establish the premise that the rest of the paper then tries to prove:
Treatments focus on reducing the number of hours viewing television behaviorally without a disease overlay such as ‘television addiction’ and are effective. This suggests a similar approach might be appropriate for high sexual desire if the proposed disease model does not add explanatory power beyond merely high sexual desire. (Steele, Staley, Fong, & Prause, 2013)
Based on this comparison, that of desire to watch TV in children and desire for sex in adults, the authors then launch into a discussion on event-related potentials (ERPs) and a subsequent description of their study design, followed by results and discussion, and culminating in the following summary:
In conclusion, the first measures of neural reactivity to visual sexual and non-sexual stimuli in a sample reporting problems regulating their viewing of similar stimuli fail to provide support for models of pathological hypersexuality, as measured by questionnaires. Specifically, differences in the P300 window between sexual and neutral stimuli were predicted by sexual desire, but not by any (of three) measures of hypersexuality. (Steele et al., 2013)
With this statement the authors put forward the premise that high desire, even if it is problematic to those who experience it, is not pathologic, no matter the consequence.
Others have described significant limitations of this study. For instance, author Nicole Prause stated in an interview, ‘Studies of drug addictions, such as cocaine, have shown a consistent pattern of brain response to images of the drug of abuse, so we predicted that we should see the same pattern in people who report problems with sex if it was, in fact, an addiction’. John Johnson has pointed out several critical issues with this use of the Dunning et al. (2011) paper she cites as a basis for comparison with the Steele et al. paper. First, the Dunning et al. paper used three controls: abstinent cocaine users, current users, and drug naïve controls. The Steele et al. paper had no control group of any kind. Second, the Dunning et al. paper measured several different ERPs in the brain, including early posterior negativity (EPN), thought to reflect early selective attention, and late positive potential (LPP), thought to reflect further processing of motivationally significant material. Furthermore, the Dunning study distinguished the early and late components of the LPP, thought to reflect sustained processing. Moreover, the Dunning et al. paper distinguished between these different ERPs in abstinent, currently using, and healthy control groups. The Steele et al. paper, however, looked only at one ERP, the p300, which Dunning compared to the early window of the LLP. The Steele et al. authors even acknowledged this critical flaw in design: ‘Another possibility is that the p300 is not the best place to identify relationships with sexually motivating stimuli. The slightly later LPP appears more strongly linked to motivation’. Steel et al. admit that they are in fact not able to compare their results to the Dunning et al. study, yet their conclusions effectively make such a comparison. Regarding the Steele et al. study, Johnson summarized, ‘The single statistically significant finding says nothing about addiction. Furthermore, this significant finding is a negative correlation between P300 and desire for sex with a partner (r=−0.33), indicating that P300 amplitude is related to lower sexual desire; this directly contradicts the interpretation of P300 as high desire. There are no comparisons to other addict groups. There are no comparisons to control groups. The conclusions drawn by the researchers are a quantum leap from the data, which say nothing about whether people who report trouble regulating their viewing of sexual images have or do not have brain responses similar to cocaine or any other kinds of addicts’ (personal communication, John A. Johnson, PhD, 2013).
Although other serious deficiencies in this study design include lack of an adequate control group, heterogeneity of study sample, and a failure to understand the limitations of the ability of the P300 to qualitatively and quantitatively discriminate and differentiate between ‘merely high sexual desire’ and pathologically unwanted sexual compulsions, perhaps the most fundamental flaw relates to the use and understanding of the term ‘desire’. It is clear that in constructing this definitional platform, the authors minimize the concept of desire with the word ‘merely’. Desire, as related to biological systems in the context of sexuality, is a complex product of mesencephalic dopaminergic drive with telencephalic cognitive and affective mediation and expression. As a primal salience factor in sex, dopamine is increasingly recognized as a key component in sexual motivation, which has been widely conserved in the evolutionary tree (Pfaus, 2010). Genes relating to both the design and expression of sexual motivation are seen across phyla and also span intra-phyla complexity. While there are obvious differences between sex, food seeking, and other behaviors, which are essential to evolutionary fitness, we now know there are similarities in the molecular machinery from which biologically beneficial ‘desire’ emanates. We now know that these mechanisms are designed to ‘learn’, in a neural connecting and modulating way. As Hebb’s law states, ‘Neurons that fire together, wire together’. We became aware of the brain’s ability to alter its structural connectivity with reward learning in early studies relating to drug addiction, but have now seen neuronal reward-based learning with such seemingly diverse natural desires relating to sex and salt craving.
Definitions relating to desire are important here; biological salience, or ‘wanting’, is one thing, whereas we consider ‘craving’ to have more ominous implications as it is used in the literature relating to drug addiction and relapse. Evidence demonstrates that craving states relating to appetites for biologically essential necessities such as salt and sex invoke – with deprivation followed by satiation – a neuroplastic process involving a remodeling and arborizing of neuronal connections (Pitchers et al., 2010; Roitman et al., 2002). Notably, a desperate desire is effected by craving states associated with conditions that portend the possible death of the organism such as salt deficiency, which induces the animal to satiate and avoid death. Drug addiction in humans, interestingly, can affect a comparable craving leading to a similar desperation to satiate in spite of the risk of death, an inversion of this elemental drive. A similar phenomenon occurs with natural addictions as well, such as the individual with morbid obesity and severe cardiac disease continuing to consume a high fat diet, or one with a sexual addiction continuing to engage in random sexual acts with strangers despite an elevated probability of acquiring sexually transmitted diseases such as HIV and hepatitis. That gene sets driving signaling cascades essential to this craving conundrum are identical for both drug addiction and the most basic of natural cravings, salt, supports a hijacking, usurping role for addiction (Liedtke et al., 2011). We also better understand how complex systems associated with and effecting these changes involve genetic molecular switches, products, and modulators such as DeltaFosB, orexin, Cdk5, neural plasticity regulator activity-regulated cytoskeleton-associated protein (ARC), striatally enriched protein tyrosine phosphatase (STEP), and others. These entities form a complex signaling cascade, which is essential to neural learning.
What we experience affectively as ‘craving’, or very ‘high desire’, is a product of mesencephalic and hypothalamic impetus which projects to, participates in, and is part of cortical processing resulting from this convergence of conscious and unconscious information. As we demonstrated in our recent PNAS paper, these natural craving states ‘likely reflect usurping of evolutionary ancient systems with high survival value by the gratification of contemporary hedonic indulgences’ (Liedtke et al., 2011, PNAS), in that we found that these same salt ‘craving’ gene sets were previously associated with cocaine and opiate addiction. The cognitive expression of this ‘desire’, this focus on getting the reward, the ‘craving’ to experience satiation again is but a conscious ‘cortical’ expression of a deeply seated and phyolgenetically primitive drive originating in the hypothalamic/mesencephalic axis. When it results in an uncontrolled and – when expressed – destructive craving for a reward, how do we split neurobiological hairs and term it ‘merely’ high desire rather than addiction?
The other issue relates to immutability. Nowhere in the Steele et al. paper is there a discussion as to why these individuals have ‘high desire’. Were they born that way? What is the role, if any, of environment on both qualitative and quantitative aspect of said desire? Can learning affect desire in at least some of this rather heterogeneous study population? (Hoffman & Safron, 2012). The authors’ perspective in this regard lacks an understanding of the process of constant modulation at both cellular and macroscopic levels. We know, for instance, that these microstructural changes seen with neuronal learning are associated with macroscopic changes as well. Numerous studies confirm the importance of plasticity, as many have compellingly argued: ‘Contrary to assumptions that changes in brain networks are possible only during critical periods of development, modern neuroscience adopts the idea of a permanently plastic brain’ (Draganski & May, 2008); ‘Human brain imaging has identified structural changes in gray and white matter that occur with learning … learning sculpts brain structure’ (Zatorre, Field, & Johansen-Berg, 2012).
Finally, consider again the author’s term ‘merely high sexual desire’. Georgiadis (2012) recently suggested a central dopaminergic role for humans in this midbrain to striatum pathway. Of all the natural rewards, sexual orgasm involves the highest dopamine spike in the striatum, with levels up to 200% of baseline (Fiorino & Phillips, 1997), which is comparable with morphine (Di Chiara & Imperato, 1988) in experimental models. To trivialize, minimize, and de-pathologize compulsive sexuality is to fail to understand the central biological role of sexuality in human motivation and evolution. It demonstrates a naiveté with regard to what is now an accepted understanding of current reward neuroscience, in that it pronounces sexual desire as inherent, immutable, and uniquely immune from the possibility of change either qualitatively or quantitatively. Even more critically, however, as illustrated by the Steele et al. paper, is that this myopic dogma fails to comprehend the truth that neuroscience now tells us that ‘high desire’, when it results in compulsive, unwanted, and destructive behavior, is ‘merely’ an addiction.
References
Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences. 1988;85(14):5274–5278. [PMC free article] [PubMed]
Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioral Brain Research. 2008;192(1):137–142. [PubMed]
Dunning J. P, Parvaz M. A, Hajcak G, Maloney T, Alia-Klein N, Woicik P. A, et al. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users: An ERP study. European Journal of Neuroscience. 2011;33(9):1716–1723. [PMC free article] [PubMed]
Fiorino D. F, Phillips A. G. Dynamic changes in nucleus accumbens dopamine efflux during the Coolidge Effect in male rats. Journal of Neuroscience. 1997;17(12):4849–4855. [PubMed]
Georgiadis J. R. Doing it … wild? On the role of the cerebral cortex in human sexual activity. Socioaffective Neuroscience and Psychology. 2012;2:17337. [PMC free article] [PubMed]
Hoffman H, Safron A. Introductory editorial to ‘The Neuroscience and Evolutionary Origins of Sexual Learning’ Socioaffective Neuroscience and Psychology. 2012;2:17415. [PMC free article] [PubMed]
Liedtke W. B, McKinley M. J, Walker L. L, Zhang H, Pfenning A. R, Drago J, et al. Relation of addiction genes to hypothalamic gene changes subserving genesis and gratification of a classic instinct, sodium appetite. Proceedings of the National Academy of Sciences. 2011;108(30):12509–12514. [PMC free article] [PubMed]
Pfaus J. G. Dopamine: Helping males copulate for at least 200 million years. Behavioral Neuroscience. 2010;124(6):877–880. [PubMed]
Pitchers K. K, Balfour M. E, Lehman M. N, Richtand N. M, Yu L, Coolen L. M. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biological Psychiatry. 2010;67:872–879. [PMC free article] [PubMed]
Roitman M. F, Na E, Anderson G, Jones T. A, Berstein I. L. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. Journal of Neuroscience. 2002;22(11):RC225: 1–5. [PubMed]
Steele V. R, Staley C, Fong T, Prause N. Sexual desire, not hypersexuality, is related to neurophysiological responses elicited by sexual images. Socioaffective Neuroscience and Psychology. 2013;3:20770. [PMC free article] [PubMed]
Winters J, Christoff K, Gorzalka B. B. Dysregulated sexuality and high sexual desire: Distinct constructs? Archives of Sexual Behavior. 2010;39(5):1029–1043. [PubMed]
Zatorre R. J, Field R. D, Johansen-Berg H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nature Neuroscience. 2012;15:528–536. [PMC free article] [PubMed]
2) Neural Correlates of Sexual Cue Reactivity in Individuals with and without Compulsive Sexual Behaviours (2014)
Excerpt critiquing Steele et al., 2013 (Citation 25 is Steele et al.)
Our findings suggest dACC activity reflects the role of sexual desire, which may have similarities to a study on the P300 in CSB subjects correlating with desire [25]. We show differences between the CSB group and healthy volunteers whereas this previous study did not have a control group. The comparison of this current study with previous publications in CSB focusing on diffusion MRI and the P300 is difficult given methodological differences. Studies of the P300, an event related potential used to study attentional bias in substance use disorders, show elevated measures with respect to use of nicotine [54], alcohol [55], and opiates [56], with measures often correlating with craving indices. The P300 is also commonly studied in substance-use disorders using oddball tasks in which low-probability targets are frequently mixed with high-probability non-targets. A meta-analysis showed that substance-use-disordered subjects and their unaffected family members had decreased P300 amplitude compared to healthy volunteers [57]. These findings suggest substance-use disorders may be characterized by impaired allocation of attentional resources to task-relevant cognitive information (non-drug targets) with enhanced attentional bias to drug cues. The decrease in P300 amplitude may also be an endophenotypic marker for substance-use disorders. Studies of event-related potentials focusing on motivation relevance of cocaine and heroin cues further report abnormalities in the late components of the ERP (>300 milliseconds; late positive potential, LPP) in frontal regions, which may also reflect craving and attention allocation [58]–[60]. The LPP is believed to reflect both early attentional capture (400 to 1000 msec) and later sustained processing of motivationally significant stimuli. Subjects with cocaine use disorder had elevated early LPP measures compared to healthy volunteers suggesting a role for early attentional capture of motivated attention along with attenuated responses to pleasant emotional stimuli. However, the late LPP measures were not significantly different from those in healthy volunteers [61]. The generators of the P300 event-related potential for target-related responses is believed to be the parietal cortex and cingulate [62]. Thus, both dACC activity in the present CSB study and P300 activity reported in a previous CSB study may reflect similar underlying processes of attentional capture. Similarly, both studies show a correlation between these measures with enhanced desire. Here we suggest that dACC activity correlates with desire, which may reflect an index of craving, but does not correlate with liking suggestive of on an incentive-salience model of addictions.
3) Neuroscience of Internet Pornography Addiction: A Review and Update (2015)
Excerpt critiquing Steele et al., 2013 (citation 303):
An EEG study on those complaining of problems regulating their viewing of internet pornography has reported the neural reactivity to sexual stimuli [303]. The study was designed to examine the relationship between ERP amplitudes when viewing emotional and sexual images and questionnaire measures of hypersexuality and sexual desire. The authors concluded that the absence of correlations between scores on hypersexuality questionnaires and mean P300 amplitudes when viewing sexual images “fail to provide support for models of pathological hypersexuality” [303] (p. 10). However, the lack of correlations may be better explained by arguable flaws in the methodology. For example, this study used a heterogeneous subject pool (males and females, including 7 non-heterosexuals). Cue-reactivity studies comparing the brain response of addicts to healthy controls require homogenous subjects (same sex, similar ages) to have valid results. Specific to porn addiction studies, it’s well established that males and females differ appreciably in brain and autonomic responses to the identical visual sexual stimuli [304, 305, 306]. Additionally, two of the screening questionnaires have not been validated for addicted IP users, and the subjects were not screened for other manifestations of addiction or mood disorders.
Moreover, the conclusion listed in the abstract, “Implications for understanding hypersexuality as high desire, rather than disordered, are discussed” [303] (p. 1) seems out of place considering the study’s finding that P300 amplitude was negatively correlated with desire for sex with a partner. As explained in Hilton (2014), this finding “directly contradicts the interpretation of P300 as high desire” [307]. The Hilton analysis further suggests that the absence of a control group and the inability of EEG technology to discriminate between “high sexual desire” and “sexual compulsion” render the Steele et al. findings uninterpretable [307].
Finally, a significant finding of the paper (higher P300 amplitude to sexual images, relative to neutral pictures) is given minimal attention in the discussion section. This is unexpected, as a common finding with substance and internet addicts is an increased P300 amplitude relative to neutral stimuli when exposed to visual cues associated with their addiction [308]. In fact, Voon, et al. [262] devoted a section of their discussion analyzing this prior study’s P300 findings. Voon et al. provided the explanation of importance of P300 not provided in the Steele paper, particularly in regards to established addiction models, concluding,
“Thus, both dACC activity in the present CSB study and P300 activity reported in a previous CSB study[303] may reflect similar underlying processes of attentional capture. Similarly, both studies show a correlation between these measures with enhanced desire. Here we suggest that dACC activity correlates with desire, which may reflect an index of craving, but does not correlate with liking suggestive of on an incentive-salience model of addictions.” [262] (p. 7)
So while these authors [303] claimed that their study refuted the application of the addiction model to CSB, Voon et al. posited that these authors actually provided evidence supporting said model.
4) Is Internet Pornography Causing Sexual Dysfunctions? A Review With Clinical Reports (2016)
Excerpt analyzing Steele et al., 2013 (citation 48):
A 2013 EEG study by Steele et al. reported higher P300 amplitude to sexual images, relative to neutral pictures, in individuals complaining of problems regulating their Internet pornography use [48]. Substance abusers also exhibit greater P300 amplitude when exposed to visual cues associated with their addiction [148]. In addition, Steele et al. reported a negative correlation between P300 amplitude and desire for sex with a partner [48]. Greater cue reactivity to Internet pornography paired with less sexual desire for partnered sex, as reported by Steele et al., aligns with the Voon et al. finding of “diminished libido or erectile function specifically in physical relationships with women” in compulsive Internet pornography users [31]. Supporting these findings, two studies assessing sexual desire and erectile function in “hypersexuals” and compulsive Internet pornography users reported associations between measures of hypersexuality, and reduced desire for partnered sex and sexual difficulties [15,30]. Additionally, the 2016 survey of 434 men who viewed Internet pornography at least once in the last three months reported that problematic use was associated with higher levels of arousabilty, yet lower sexual satisfaction and poorer erectile function [44]. These results should be viewed in light of the multiple neuropsychology studies that have found that sexual arousal to Internet pornography cues and cravings to view pornography were related to symptom severity of cybersex addiction and self-reported problems in daily life due to excessive Internet pornography use [52,53,54,113,115,149,150]. Taken together, multiple and varied studies on Internet pornography users align with the incentive-salience theory of addiction, in which changes in the attraction value of an incentive correspond with changes in activation of regions of the brain implicated in the sensitization process [31,106]. To sum up, in alignment with our hypothesis, various studies report that greater reactivity toward pornographic cues, cravings to view, and compulsive pornography use are associated with sexual difficulties and diminished sexual desire for partners.
5) Conscious and Non-Conscious Measures of Emotion: Do They Vary with Frequency of Pornography Use? (2017)
YBOP COMMENTS: This 2017 EEG study on porn users cited 3 Nicole Prause EEG studies. The authors believe that all 3 Prause EEG studies actually found desensitization or habituation in frequent porn users (which often occurs with addiction). This is exactly what YBOP has always claimed (explained in this critique: Critique of: Letter to the editor “Prause et al. (2015) the latest falsification of addiction predictions” 2016).
In the excerpts below these 3 citations indicate the following Nicole Prause EEG studies (#14 is Steele et al., 2013):
- 7 – Prause, N.; Steele, V.R.; Staley, C.; Sabatinelli, D. Late positive potential to explicit sexual images associated with the number of sexual intercourse partners. Soc. Cogn. Affect. Neurosc. 2015, 10, 93–100.
- 8 – Prause, N.; Steele, V.R.; Staley, C.; Sabatinelli, D.; Hajcak, G. Modulation of late positive potentials by sexual images in problem users and controls inconsistent with “porn addiction”. Biol. Psychol. 2015, 109, 192–199.
- 14 – Steele, V.R.; Staley, C.; Fong, T.; Prause, N. Sexual desire, not hypersexuality, is related to neurophysiological responses elicited by sexual images. Socioaffect. Neurosci. Psychol. 2013, 3, 20770
Excerpts describing Steele et al., 2013:
Event-related potentials (ERPs) have often been used as a physiological measure of reactions to emotional cues, e.g., [24]. Studies utilizing ERP data tend to focus on later ERP effects such as the P300 [14] and Late-Positive Potential (LPP) [7, 8] when investigating individuals who view pornography. These later aspects of the ERP waveform have been attributed to cognitive processes such as attention and working memory (P300) [25] as well as sustained processing of emotionally-relevant stimuli (LPP) [26]. Steele et al. [14] showed that the large P300 differences seen between viewing of sexually explicit images relative to neutral images was negatively related to measures of sexual desire, and had no effect on participants’ hypersexuality. The authors suggested that this negative finding was most probably due to the images shown not having any novel significance to the participant pool, as participants all reported viewing high volumes of pornographic material, consequently leading to the suppression of the P300 component. The authors went on to suggest that perhaps looking at the later occurring LPP may provide a more useful tool, as it has been shown to index motivation processes. Studies investigating the effect pornography use has on the LPP have shown the LPP amplitude to be generally smaller in participants who report having higher sexual desire and problems regulating their viewing of pornographic material [7, 8]. This result is unexpected, as numerous other addiction-related studies have shown that when presented with a cue-related emotion task, individuals who report having problems negotiating their addictions commonly exhibit larger LPP waveforms when presented images of their specific addiction-inducing substance [27]. Prause et al. [7, 8] offer suggestions as to why the use of pornography may result in smaller LPP effects by suggesting that it may be due to a habituation effect, as those participants in the study reporting overuse of pornographic material scored significantly higher in the amount of hours spent viewing pornographic material.
———–
Studies have consistently shown a physiological downregulation in processing of appetitive content due to habituation effects in individuals who frequently seek out pornographic material [3, 7, 8]. It is the authors’ contention that this effect may account for the results observed.
————
Future studies may need to utilise a more up-to-date standardised image database to account for changing cultures. Also, maybe high porn users downregulated their sexual responses during the study. This explanation was at least used by [7, 8] to describe their results which showed a weaker approach motivation indexed by smaller LPP (late positive potential) amplitude to erotic images by individuals reporting uncontrollable pornography use. LPP amplitudes have been shown to decrease upon intentional downregulation [62, 63]. Therefore, an inhibited LPP to erotic images may account for lack of significant effects found in the present study across groups for the “erotic” condition.
———–
6) Neurocognitive mechanisms in compulsive sexual behavior disorder (2018).
Excerpts analyzing Steele et al., 2013 (which is citation 68):
Klucken and colleagues recently observed that participants with CSB as compared to participants without displayed greater activation of the amygdala during presentation of conditioned cues (colored squares) predicting erotic pictures (rewards) [66]. These results are like those from other studies examining amygdala activation among individuals with substance use disorders and men with CSB watching sexually explicit video clips [1, 67]. Using EEG, Steele and colleagues observed a higher P300 amplitude to sexual images (when compared to neutral pictures) among individuals self-identified as having problems with CSB, resonating with prior research of processing visual drug cues in drug addiction [68, 69].
YBOP comments: In the above excerpt the authors of the current review are saying that Steele et al’s findings indicate cue-reactivity in frequent porn users. This aligns with the addiction model and cue-reactivity is a neuro-physiological marker for addiction. While Steele et al. spokesperson Nicole Prause claimed that the subjects’ brain response differed from other types of addicts (cocaine was the example given by Prause) – this was not true, and not reported anywhere in Steele et al., 2013
————-
Furthermore, habituation may be revealed through decreased reward sensitivity to normally salient stimuli and may impact reward responses to sexual stimuli including pornography viewing and partnered sex [1, 68]. Habituation has also been implicated in substance and behavioral addictions [73-79].
YBOP comments: In the above excerpt the authors of this review are referring to Steele et al’s finding of greater cue-reactivity to porn related to less desire for sex with a partner (but not lower desire to masturbate to porn). To put another way – individuals with more brain activation and cravings related to porn preferred to masturbate to porn than have sex with a real person. That’s less reward sensitivity to “partnered sex”, which is “normally salient stimuli”. Together these two Steele et al. findings indicate greater brain activity to cues (porn images), yet less reactivity to natural rewards (sex with a person). Both are hallmarks of an addiction.
7) Online Porn Addiction: What We Know and What We Don’t—A Systematic Review (2019)
Excerpt critiquing Steele et al., 2013 (citation 105 is Steele et al.)
Evidence of this neural activity signalizing desire is particularly prominent in the prefrontal cortex [101] and the amygdala [102,103], being evidence of sensitization. Activation in these brain regions is reminiscent of financial reward [104] and it may carry a similar impact. Moreover, there are higher EEG readings in these users, as well as the diminished desire for sex with a partner, but not for masturbation to pornography [105], something that reflects also on the difference in erection quality [8]. This can be considered a sign of desensitization. However, Steele’s study contains several methodological flaws to consider (subject heterogeneity, a lack of screening for mental disorders or addictions, the absence of a control group, and the use of questionnaires not validated for porn use) [106]. A study by Prause [107], this time with a control group, replicated these very findings. The role of cue reactivity and craving in the development of cybersex addiction have been corroborated in heterosexual female [108] and homosexual male samples [109].
8) The Initiation and Development of Cybersex Addiction: Individual Vulnerability, Reinforcement Mechanism and Neural Mechanism (2019)
Excerpt critiquing Steele et al., 2013:
First, Steele et al. (2013) found that individuals with viewing of visual sexual stimuli (VSS) induced a greater amplitude of the P300 component when viewing erotic images than when viewing neutral images. The results seem to confirm the notion that online pornography leads to an individual’s hunger for online pornography, but Steele’s research lacks normal subjects for reference. In addition, LPP components appear later than P300. Late positive potential is associated with the stimulation of significant material processing and better reflects the individual’s desire to watch pornographic material (Hilton, 2014) (the greater the individual’s desire to watch pornography, the greater the LPP volatility). In this regard, Prause and Steele et al. (2015) added individuals who viewed less pornographic material to VSS individuals in the improvement experiment, and found that subjects who had excessively viewed pornographic material problems and reported more sexual desire were watching erotic images. The induced LPP amplitude is smaller, and this result seems to be contrary to the idea that online pornography-related clues induce a sense of craving. Actually, some scholars have pointed out that the erotic images used in the study by Prause and Steele may be an addiction in itself. Consumer goods, not addictive cues (Gola et al., 2017; Gola, Wordecha, Marchewka, & Sescousse, 2016). Therefore, according to the Theory of Incentive-Salience Theory (IST) in drug addiction, as the degree of addiction deepens, the cues of addiction can induce the addicted desire of addicted individuals to become more and more addicted. (Berridge, 2012; Robinson, Fischer, Ahuja, Lesser, & Maniates, 2015), but the addiction to the addicted individuals has gradually decreased, and the decrease in LPP amplitude indicates that CA may be addicted to drugs.

One thought on “Peer-reviewed critiques of Steele et al., 2013”
Comments are closed.