Biol Psychiatry. 2008 Dec 1;64(11):941-50. Epub 2008 Jul 26.
Teegarden SL, Nestler EJ, Bale TL.
Source
Department of Animal Biology, University of Pennsylvania, Philadelphia, PA 19104-6046, USA.
Abstract
BACKGROUND:
Sensitivity to reward has been implicated as a predisposing factor for behaviors related to drug abuse as well as overeating. However, the underlying mechanisms contributing to reward sensitivity are unknown. We hypothesized that a dysregulation in dopamine signaling might be an underlying cause of heightened reward sensitivity whereby rewarding stimuli could act to normalize the system.
METHODS:
We used a genetic mouse model of increased reward sensitivity, the Delta FosB-overexpressing mouse, to examine reward pathway changes in response to a palatable high-fat diet. Markers of reward signaling in these mice were examined both basally and following 6 weeks of palatable diet exposure. Mice were examined in a behavioral test following high-fat diet withdrawal to assess the vulnerability of this model to removal of rewarding stimuli.
RESULTS:
Our results demonstrate altered reward pathway activation along the nucleus accumbens-hypothalamic-ventral tegmental area circuitry resulting from overexpression of Delta FosB in the nucleus accumbens and striatal regions. Levels of phosphorylated cyclic adenosine monophosphate (cAMP) response element binding protein (pCREB), brain-derived neurotrophic factor (BDNF), and dopamine and cyclic adenosine monophosphate regulated phosphoprotein with a molecular mass of 32 kDa (DARPP-32) in the nucleus accumbens were reduced in Delta FosB mice, suggestive of reduced dopamine signaling. Six weeks of high-fat diet exposure completely ameliorated these differences, revealing the potent rewarding capacity of a palatable diet. Delta FosB mice also showed a significant increase in locomotor activity and anxiety-related responses 24 hours following high-fat withdrawal.
CONCLUSIONS:
These results establish an underlying sensitivity to changes in reward related to dysregulation of Delta FosB and dopamine signaling that can be normalized with palatable diets and may be a predisposing phenotype in some forms of obesity.
Introduction
Despite our increasing knowledge of the neural systems that control appetite and satiety, rates of obesity continue to rise in the United States. Current drug treatments have limited efficacy, and behavioral modifications suffer from minimal long-term compliance (1). The consumption of calorically-dense, palatable foods has been linked to changes in stress and reward pathways in the brain, suggesting that the rewarding properties of such foods may override energy balance signals (2-4). Foods high in fat act as natural rewards, activating brain reward centers in a manner similar to drugs of abuse, and as such have been used in self-administration paradigms (5-8). Thus, it is likely that behaviors and motivation for overeating and drug abuse share common underlying mechanisms, potentially opening up new avenues of treatment for both conditions.
In studying the relationship between palatable foods and pathways regulating reward and stress in the brain, we have previously identified molecular and biochemical markers of reduced reward and increased stress following withdrawal from a palatable high fat diet (HF). Similar to drugs of abuse, exposure to a palatable diet in our studies resulted in increased levels of the transcription factor ΔFosB in the nucleus accumbens (NAc), a central brain reward structure (9, 10). Mice that inducibly overexpress ΔFosB show increased instrumental responding for a food reward (11), making them a valuable tool for examining the role of reward sensitivity and long-term dysregulation of the reward system in the molecular and biochemical responses to a palatable diet.
In the present study, we utilized the ΔFosB-overexpressing mice to examine long-term alterations in markers of reward in the NAc-hypothalamus–ventral tegmental area (VTA) neurocircuitry in response to a palatable HF diet. Based upon previous studies in these reward-sensitive mice, we hypothesized that ΔFosB-induced changes in reward sensitivity involve a dysregulation in dopamine signaling resulting from NAc feedback to the VTA. Further, we hypothesized that exposure to a natural reward of an energy-dense HF diet would then normalize the dopaminergic system in these mice, resulting in an exaggerated response to the stress of withdrawal from this HF diet. The unique aspect of utilizing a palatable diet as a rewarding substance allows us to include the hypothalamic inputs to reward circuitry in a phenotype that may be predictive of a population predisposed to treatment-resistant obesity. To examine this hypothesis, we studied markers of dopamine neurotransmission, including pCREB, BDNF, and DARPP-32 in the NAc and tyrosine hydroxylase and the dopamine transporter in the VTA, following HF exposure. We also examined specific markers of energy balance known to influence dopamine output, including leptin and orexin receptors in the VTA and orexin expression within the lateral hypothalamus.
Materials and Methods
Animals
Male bitransgenic mice that inducibly overexpress ΔFosB in dynorphin-positive neurons in the NAc and dorsal striatum (Kelz et al., 1999) were generated on a mixed background (ICR:C57Bl6/SJL) at The University of Texas Southwestern Medical Center and maintained and tested at the University of Pennsylvania. All mice were maintained on doxycycline (100 μg/ml in the drinking water) until arrival at the University of Pennsylvania. To induce overexpression, doxycycline was removed (n = 23)(12). Control mice (n = 26) continued to receive the drug. Mice were assigned to diet groups eight weeks following the removal of doxycyline at which time expression has been shown to reach maximal levels (13). Mice were maintained on a 12:12 light-dark cycle (lights on 0700) with food and water available ad libitum. All studies were performed according to experimental protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee, and all procedures were conducted in accordance with institutional guidelines.
Diet exposure
Mice were maintained on house chow (n=16) or placed on HF (n = 16-17) for six weeks. House chow (Purina Lab Diet, St. Louis, MO) contained 4.00 kcal/g, consisting of 28% protein, 12% fat, and 60% carbohydrate. HF diet (Research Diets, New Brunswick, NJ) contained 4.73 kcal/g, consisting of 20% protein, 45% fat, and 35% carbohydrate.
Biochemistry and gene expression
Mice were analyzed after six weeks of diet exposure. Brains were removed from the skull and either frozen whole on dry ice or the NAc dissected (approximately 0.5 – 1.75 mm from bregma, at a depth of 3.5 – 5.5 mm) and frozen in liquid nitrogen. Tissue was stored at -80°C until assayed.
Biochemical analyses
Methods for Western blots are described in supplemental materials. The antibodies used were: Cdk5, CREB, and BDNF (1:500, Santa Cruz Biotechnology, Santa Cruz, CA) and phospho-CREB (pCREB) (Ser 133) (1:500, Cell Signaling Technology, Danvers, MA).
Receptor autoradiography
Detailed methods for autoradiography are described in supplemental materials. Ligands used were 2 nM H3 – SCH 23390 and 5 nM H3 – spiperone (PerkinElmer, Boston, MA).
In situ hybridization
Tissue processing and hybridization were performed as previously described (14). The DARPP-32 probe was kindly provided by P. Greengard (Rockefeller University), and the orexin probe by J. Elmquist (The University of Texas Southwestern Medical Center). Slides assayed for DARPP-32 were apposed to film for 3 days, and slides assayed for orexin were apposed to film for 4 days. Quantification of film images was conducted as previously described (10).
QRT-PCR
RNA was isolated from the VTA and expression of individual genes assessed using TaqMan gene expression assays (Applied Biosystems, Foster City, CA). Detailed methods and statistical analyses may be found in supplemental materials.
Behavioral analyses
In order to examine the effects of reward sensitivity on diet-induced behavioral changes, a subset of mice was withdrawn from HF following four weeks of exposure and returned to house chow (n = 9 control, n = 8 ΔFosB). Twenty-four hours following withdrawal, mice were exposed to the open-field test in accordance with our previously published dietary withdrawal paradigm (10). Briefly, the mouse was placed in the center of the open-field apparatus and monitored for five minutes. Total line crosses, fecal boli, time in the center, and crosses into the center were measured.
Statistics
All data except Western blots were analyzed using a two-way ANOVA followed by Fisher’s PLSD test with doxycycline treatment (ΔFosB expression) and diet condition as the independent variables. For RT-PCR analyses, a reduced P value was utilized to correct for multiple comparisons within groups of related genes (see supplemental materials). Western blots were analyzed using a student’s t-test with doxycycline treatment as the independent variable, comparing optical densities within the same blot. All data are presented as mean ± SEM.
Results
Basal biochemical differences
To elucidate the molecular pathways that underlie the enhanced reward sensitivity in ΔFosB-overexpressing mice, levels of several key signaling molecules were examined in the NAc. There was a trend for increased levels of Cdk5 in the NAc of ΔFosB mice compared to littermate control animals maintained on doxycycline (F = 5.1, P = 0.08; Fig. 1A). ΔFosB mice expressed significantly reduced levels of pCREB (F = 7.4, P < 0.05; Fig. 1B) as well as total levels of CREB (F = 5.4, P = 0.05; Fig. 1C). A significant reduction in BDNF was also observed in the NAc of ΔFosB mice (F = 10.6, P < 0.05; Fig. 1D).
Figure 1
Mice overexpressing ΔFosB exhibited biochemical markers of reduced dopamine signaling in the NAc
Food intake and body weight on high fat diet
We next examined the effects of a naturally rewarding HF diet on alterations in signaling molecules in the ΔFosB-overexpressing mice. There were no differences between ΔFosB mice and controls in food intake on either house or HF. However, there was an overall decrease in caloric intake normalized to body weight when exposed to HF that was specific to the ΔFosB mice (F = 11.2, P < 0.01; Fig. 2A). At the end of six weeks of diet exposure, mice receiving HF weighed significantly more than those on chow diet (F = 17.2, P < 0.001), and ΔFosB mice weighed less overall than controls (F = 5.6, P < 0.05; Fig. 2B). This effect was specific to differences between groups on the chow diet (P < 0.05).
Figure 2
ΔFosB overexpressing mice showed no differences in food intake on either chow or high fat (HF) diet
Biochemical differences on high fat diet
To determine how basal differences in NAc signaling might be altered by HF diet, the same signaling proteins studied at baseline were examined in animals that had received six weeks of HF. There were no significant differences in Cdk5 levels (Fig. 3A). Levels of pCREB and total CREB were no longer different after six weeks of HF (Fig. 3B,C). Levels of BDNF were significantly elevated in ΔFosB mice following six weeks of HF exposure (F = 6.5, P = 0.05; Fig. 3D).
Figure 3
High fat (HF) diet ameliorated signaling differences observed in the NAc of ΔFosB overexpressing mice
Dopamine receptor autoradiography
We used receptor autoradiography to assess whether the ΔFosB-induced alterations in dopamine signaling in the NAc are related to changes in dopamine receptor expression (Fig. 4A). High fat diet appeared to increase slightly the density of D1 dopamine receptor binding (P = 0.14), and this difference was greater in ΔFosB mice (Fig. 4B). There was also a trend toward an increase in D1 binding area following HF (P = 0.06), and post hoc testing showed this to be significant in the ΔFosB mice (P < 0.05; Fig. 4C). In contrast to D1 receptors, no changes in D2 receptor binding density (control chow = 97.6 ± 6.9, control HF = 101.1 ± 8.2, ΔFosB chow = 91.6 ± 1.0, ΔFosB HF = 94.8 ± 9.5) or binding area (control chow = 47.3 ± 3.4, control HF = 53.8 ± 6.0, ΔFosB chow = 51.9 ± 3.7, ΔFosB HF = 49.0 ± 3.3) in the NAc were observed.
Figure 4
High fat diet (HF) led to changes in D1 dopamine receptor binding and DARPP-32 expression in the nucleus accumbens (NAc) of ΔFosB overexpressing mice
DARPP-32 expression in the NAc
In situ hybridization was used to determine expression levels of DARPP-32 in the NAc (Fig. 4D). High fat diet significantly increased DARPP-32 expression in this brain region (F = 5.1, P < 0.05), and there was a significant interaction between diet and ΔFosB expression (F = 8.9, P < 0.05), with ΔFosB mice showing a greater diet-induced change (Fig. 4E). A basal difference in DARPP-32 expression between control and ΔFosB mice was revealed by post hoc testing (P < 0.01), as well as a significant increase in DARPP-32 expression in the ΔFosB mice on HF (P < 0.01).
Gene expression in the VTA
QRT-PCR was utilized to assess changes in gene expression in the VTA, targeting several key genes previously implicated in the regulation of reward. All samples were normalized to β-actin. To ensure that β-actin expression was not altered by treatment, a separate assay was run to compare β-actin to a second internal control, GAPDH. There were no significant differences in β-actin expression (ΔCT values, β-actin – GAPDH: control chow = 2.29 ± 0.21, control HF = 2.01 ± 0.04, ΔFosB chow = 2.32 ± 0.49, ΔFosB HF = 2.37 ± 0.10).
A trend for an interaction between ΔFosB expression and diet treatment was observed for expression of tyrosine hydroxylase (F = 3.6, P < 0.06; Fig. 5A). Six weeks of exposure to HF appeared to decrease tyrosine hydroxylase expression in control mice and increase expression in ΔFosB mice. A significant interaction between ΔFosB expression and diet exposure was observed for expression of the dopamine transporter (F = 6.7, P < 0.03; Fig. 5B). Similar to tyrosine hydroxylase, exposure to HF reduced dopamine transporter expression in control mice and significantly increased expression in ΔFosB mice (P < 0.05). The basal difference in dopamine transporter expression between control and ΔFosB mice did not reach significance (P = 0.16), but after 6 wks of HF, ΔFosB mice expressed significantly elevated levels of dopamine transporter compared to controls (P < 0.05).
Figure 5
High fat diet (HF) exposure and ΔFosB expression led to changes in expression of a number of key molecules in the VTA
There was a trend indicating an effect of increased ΔFosB expression to reduce TrkB levels in the VTA (F = 5.7, P < 0.04; Fig. 5C). Although there were no main effects on κ-opioid receptor expression, there was a trend toward reduced expression in ΔFosB mice (P = 0.08; Fig. 5D). Expression of the leptin receptor was also determined in the VTA. A significant effect of diet exposure was found (F = 6.1, P < 0.03), with HF significantly reducing levels of the leptin receptor in the VTA in both ΔFosB and control mice (Fig. 5E). Expression of orexin receptor 1 in the VTA was also examined. There was a significant effect of diet on expression of the orexin receptor (F = 9.0, P < 0.02), with mice exposed to HF expressing higher levels in the VTA (Fig. 5F). There was also a trend for ΔFosB mice to express overall higher levels of orexin receptor 1 in this brain region (P < 0.05).
Orexin expression in the lateral hypothalamus
We measured levels of orexin in the lateral hypothalamus, the origin of orexinergic innervation of the VTA, by in situ hybridization (Fig. 6A). There was a significant interaction between ΔFosB expression and diet exposure on orexin expression (F = 9.1, P < 0.01), with HF significantly increasing orexin levels in control mice (P < 0.05) and decreasing expression in ΔFosB mice (Fig. 6B). Although there were no significant differences in orexin expression in the basal state, following 6 wks of HF, ΔFosB mice expressed significantly reduced levels of orexin compared to controls (P < 0.05).
Figure 6
High fat (HF) diet had differential effects on orexin expression in control (Ctrl) and ΔFosB overexpressing mice
Behavioral Analyses
To evaluate alterations in arousal and emotionality due to dietary change, mice were exposed to the open-field test 24 hrs after withdrawal of the HF diet (10). Total line crosses, which were scored as a measure of arousal, were significantly affected by ΔFosB expression (F = 6.6, P < 0.05) and diet (F = 4.6, P < 0.05; Fig. 7A). ΔFosB mice were more active in the novel environment than contrils, and post hoc testing showed that mice withdrawn from HF were significantly more active than those exposed to chow (P < 0.05). Fecal boli were counted as a measure of anxiety-like behavior (10). There was a main effect of ΔFosB expression (F = 10.2, P < 0.01), with ΔFosB-overexpressing mice producing more fecal boli in the novel environment, particularly in the house chow and HF withdrawal groups (Fig. 7B). ΔFosB mice maintained on HF diet produced fewer fecal boli than those maintained on chow and those withdrawn 24 hrs prior to the test. Control mice did not appear to be affected by diet. There were no significant effects of either ΔFosB expression or diet on time spent in the center of the open field (control chow = 14.5 ± 3.1 sec, control HF = 18.0 ± 3.2 sec, control W/D = 15.4 ± 1.9 sec, ΔFosB chow = 16.9 ± 2.4 sec, ΔFosB HF = 13.1 ± 3.9 sec, ΔFosB W/D = 19.8 ± 2.6 sec).
Figure 7
Mice over-expressing ΔFosB were more sensitive to the effects of high fat diet (HF) withdrawal
Discussion
In obesity treatment, there is a critical need for identification of factors that influence susceptibility to overeating and weight gain. Brain reward pathways play an important role in the motivation for and response to palatable foods and dietary changes (6, 10, 15, 16). As orexigenic and anorexigenic signals can directly influence reward signaling via a hypothalamus-VTA-NAc circuit, elucidation of genes responsive to energy-rich palatable diets within reward centers may provide novel therapeutic targets in obesity treatment (17, 18). Therefore, we examined biochemical and molecular markers of reward and energy balance signaling along the hypothalamus-VTA-NAc circuit in response to a HF diet in ΔFosB-overexpressing mice as a model of enhanced sensitivity to changes in reward (13, 19, 20), and the behavioral sensitivity following diet withdrawal. We hypothesized that basal dysregulation of dopamine signaling in ΔFosB mice would be normalized by the rewarding effects of a HF diet, encompassing the intersection of energy balance signals and the dopamine system.
To examine markers indicative of a dysregulation in dopamine signaling in the NAc, we examined D1 receptor levels and downstream effectors. Although there were no significant differences in D1 receptor binding, there was a trend for HF exposure to increase binding area in the ΔFosB mice. This is interesting as induction of ΔFosB by drug and natural rewards appears to predominate in the dynorphin-positive subtype of medium spiny neurons that primarily express D1 receptors (9, 21). Levels of the downstream dopamine signaling target pCREB were significantly reduced in ΔFosB mice, supportive of reduced D1 receptor activation in this brain region (22, 23). Interestingly, we also detected a significant decrease in total CREB levels in ΔFosB mice, suggesting a further reduced capacity for dopamine signal transduction that may be secondary to feedback resulting from a prolonged decrease in pCREB (24). BDNF expression is regulated by pCREB, is elevated with D1 activation, and is an important mediator of reward-related neuroplasticity in the NAc (25, 26). Accordingly, we detected a significant decrease in BDNF protein in the NAc of ΔFosB mice.
All medium spiny neurons in the NAc express DARPP-32 (27). Its numerous downstream effectors make it a vital player in reward pathways (28), and it has been implicated in drug addiction and in other disorders involving the dopamine system including affective disorders and schizophrenia (27, 29). We detected profound basal reductions in DARPP-32 expression in the NAc of ΔFosB mice. DARPP-32 expression is regulated by BDNF, and therefore the reduced expression may be directly related to the reductions in BDNF levels detected in ΔFosB mice (27, 29, 30). Even moderate changes in the phosphorylation state of DARPP-32 can lead to substantial alterations in intracellular signaling within the NAc (27). Previous studies have reported no change in DARPP-32 protein in ΔFosB mice following a 12-wk removal from doxycycline when a more broad striatal assessment was conducted (31), suggesting that effects of ΔFosB on DARPP-32 may be time and region specific.
We hypothesized that the dramatic reductions in indices of dopamine signaling in the NAc of ΔFosB mice likely involved changes in the VTA dopamine projection neurons, even though ΔFosB is not overexpressed within these neurons. Therefore, we examined expression of dopamine-related genes in the VTA, including tyrosine hydroxylase and the dopamine transporter. Levels of tyrosine hydroxylase and dopamine transporter are positively correlated with dopamine output. There was a trend for ΔFosB mice to exhibit reduced tyrosine hydroxylase and a significant reduction in dopamine transporter, in keeping with the dysregulation of dopamine signaling in the NAc. As these basal reductions in dopamine-related genes in the VTA of ΔFosB mice presumably reflect altered feedback from the NAc during long-term ΔFosB overexpression, we examined expression of the BDNF receptor, TrkB, as a possible mechanism of NAc feedback to the VTA (32). Similar to tyrosine hydroxylase and dopamine transporter, TrkB expression also showed a trend to be basally reduced in ΔFosB mice that did not reach significance when corrected for multiple comparisons. The BDNF-TrkB complex can be retrogradely transported and act within the VTA to affect local gene expression and promote cell growth and maintenance (33). Further, BDNF activation of presynaptic TrkB within the NAc can directly stimulate dopamine neurotransmission (32), supporting an underlying diminution of dopamine signaling in these mice.
Dynorphin activation of κ-opioid receptors regulates dopamine signaling and is another mechanism whereby the NAc provides feedback to the VTA (34). We found that κ-opioid receptor expression in the VTA showed a trend to be reduced in ΔFosB mice. As ΔFosB overexpression has been shown to decrease dynorphin expression in the NAc (20), ΔFosB mice likely have profound reductions in net VTA κ-opioid activation. Although dynorphin signaling normally exerts an inhibitory effect on dopamine neurons (35), rats that show enhanced self-administration of drugs of abuse exhibit reduced levels of dynorphin in the NAc, pointing to a role for basally reduced dynorphin signaling in enhancing reward sensitivity (36, 37). Dysregulation of the dynorphin – κ-opioid system has been linked to the acquisition and persistence of drug abuse, supporting a critical balance of opioid signaling in normalization of dopamine pathways (38).
Based on the rewarding capacity of an energy-dense HF diet, we hypothesized that a dysregulation in dopamine and opioid reward signaling in ΔFosB mice would predispose these mice to enhanced reward responses to such a diet, thus normalizing the reward system via activation of the hypothalamus-VTA-NAc circuit. During the six week diet exposure, no differences in food intake between ΔFosB and control mice were observed, suggesting that the changes found in biochemical and molecular markers of reward signaling in ΔFosB mice were not due to differences in the calories consumed. As anticipated, basal differences detected in pCREB, total CREB, BDNF, DARPP-32, and κ-opioid receptor levels between ΔFosB and control mice were attenuated, likely due to increased dopamine output in ΔFosB mice on HF (29, 39-41).
Examination of both tyrosine hydroxylase and the dopamine transporter in the VTA revealed surprising opposing responses of ΔFosB and control mice following HF. Control mice showed a decrease in tyrosine hydroxylase and dopamine transporter expression, while ΔFosB mice displayed increased expression of both of these dopamine-related genes. Interestingly, tyrosine hydroxylase expression is altered in the VTA by chronic cocaine or methamphetamine administration (42-44), suggesting that ΔFosB mice may find the natural reward of HF more salient than control mice.
In order to examine how potential hypothalamic input to the VTA may be relaying signals that reflect energy balance, expression of the leptin receptor and orexin receptor-1 were also examined. Circulating leptin levels are increased by HF, and leptin can in turn act at the VTA to alter dopamine signaling (18, 45). VTA leptin receptor expression was similarly decreased by HF in both ΔFosB and control mice, in keeping with similar weight gain and diet intake while on HF. High fat also increased expression of the orexin receptor-1 in the VTA of both ΔFosB and control mice. Orexin activates dopamine neurons in the VTA, promotes VTA plasticity, and increases dopamine levels in the NAc (46-48). High fat diet has been shown to increase orexin expression in mice, in accordance with our observations (49, 50). Thus, increased expression of the orexin receptor as well as changes in leptin signaling in the VTA could promote diet reward in both ΔFosB and control mice, supporting a dissociation between pathways relaying energy balance signals and those tied directly to reward.
To examine the stress-provoking effects of reward withdrawal, mice were examined in an open-field test 24 hrs following the removal of HF. ΔFosB mice were more sensitive to the acute effects of preferred diet withdrawal, showing heightened arousal activity and fecal boli production in the novel open arena compared to all other control and diet groups. ΔFosB mice also showed an interesting behavioral pattern in this test suggestive of reward and stress sensitivity, with the HF diet initially reducing fecal boli production relative to chow, and the withdrawal again increasing this anxiety-related response. This observed increase in open-field activity did not correlate with changes in orexin expression, suggesting a relationship to stress-induced arousal that is not merely an effect of changes in orexin-mediated signaling. Overall, these data support our hypothesis that ΔFosB mice would be more sensitive to the acute effects of preferred diet withdrawal due to their heightened reward sensitivity.
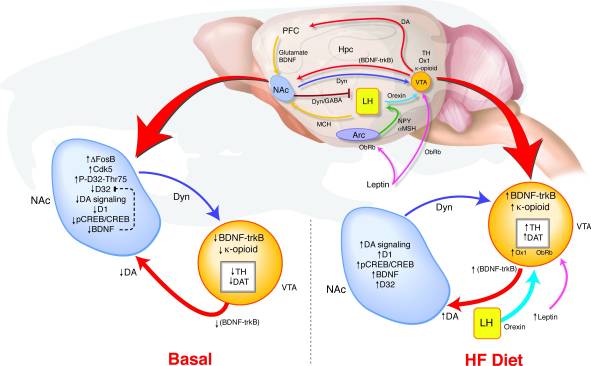
How does long-term overexpression of ΔFosB in the NAc lead to such changes in behavior and reward signaling? We have proposed a model of VTA coincident detection wherein altered feedback from the NAc and hypothalamus relays signals regarding reward state to determine the regulation of the dopamine system that may support a link between reward pathway dysregulation and a predisposition to obesity (Fig. 8). During HF exposure, multiple inputs reflecting both energy balance and reward state converge on the VTA. Increases in leptin and orexin signaling as well as altered feedback from the NAc to the lateral hypothalamus may affect how these orexigenic signals respond to HF in the ΔFosB mice (17, 18, 45, 47, 51-53). High fat diet-induced elevations in BDNF may provide reward feedback to the VTA, further promoting changes in dopamine-related gene expression.
Figure 8
High fat (HF) diet normalizes dysregulated reward signaling in ΔFosB mice
These results delineate molecular markers of reward sensitivity and indicate that long-term dysregulation of the dopamine system may predispose an individual to addiction and obesity. Further, these data provide an important step toward pinpointing potential new therapeutic targets in the treatment and prevention of obesity and other disorders that may center on the reward system. In the future, it will be important to investigate how this system responds to removal of the HF diet, as well as to investigate any sex differences in sensitivity to reward and high fat diet exposure.
Supplementary Material
Supp. Methods
Click here to view.(61K, doc)
Acknowledgments
The authors wish to thank Cathy Steffen for assistance with animal breeding and transfer. This work was supported by a grant from the University of Pennsylvania Diabetes Center (DK019525) and by grants from the National Institute of Mental Health (R01 MH51399 and P50 MH66172) and National Institute on Drug Abuse (R01 DA07359).
Footnotes
Financial Disclosures: All authors declare that they have no biomedical financial interests or potential conflicts of interest.
References
1. Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20.[PubMed]
2. Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, Lerman C. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology (Berl) 2005
3. Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45(2):198–201.[PubMed]
4. Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–11.[PubMed]
5. Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31(7):1362–70.[PubMed]
6. Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1236–9.[PubMed]
7. Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133(1):293–303.[PubMed]
8. Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–45.[PubMed]
9. Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98(20):11042–6. [PMC free article][PubMed]
10. Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61(9):1021–9.[PubMed]
11. Olausson P, Jentsch JD, Tronson N, Nestler EJ, Taylor JR. dFosB in the Nucleus Accumbens Regulates Food-Reinforced Instrumental Behavior and Motivation. The Journal of Neuroscience. 2006;26(36):9196–9204.[PubMed]
12. Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, Picciotto MR, Duman RS, Nestler EJ. Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol. 1998;54(3):495–503.[PubMed]
13. Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401(6750):272–6.[PubMed]
14. Bale TL, Dorsa DM. Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology. 1995;136(1):27–32.[PubMed]
15. Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84(3):359–62.[PubMed]
16. Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23(7):2882–8.[PubMed]
17. Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27(41):11075–82.[PubMed]
18. Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–10.[PubMed]
19. Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23(6):2488–93.[PubMed]
20. Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9(2):205–11.[PubMed]
21. Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103(9):3399–404. [PMC free article][PubMed]
22. Blendy JA, Maldonado R. Genetic analysis of drug addiction: the role of cAMP response element binding protein. J Mol Med. 1998;76(2):104–10.[PubMed]
23. Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47 1:24–32.[PubMed]
24. Tanis KQ, Duman RS, Newton SS. CREB Binding and Activity in Brain: Regional Specificity and Induction by Electroconvulsive Seizure. Biol Psychiatry. 2007
25. Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–14.[PubMed]
26. Graham DL, Edwards S, Bachtell RK, Dileone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10(8):1029–37.[PubMed]
27. Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. Aaps J. 2005;7(2):E353–60. [PMC free article][PubMed]
28. Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16(5):291–305.[PubMed]
29. Bogush A, Pedrini S, Pelta-Heller J, Chan T, Yang Q, Mao Z, Sluzas E, Gieringer T, Ehrlich ME. AKT and CDK5/p35 mediate brain-derived neurotrophic factor induction of DARPP-32 in medium size spiny neurons in vitro. J Biol Chem. 2007;282(10):7352–9.[PubMed]
30. Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann N Y Acad Sci. 2004;1025:335–44.[PubMed]
31. Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410(6826):376–80.[PubMed]
32. Blochl A, Sirrenberg C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J Biol Chem. 1996;271(35):21100–7.[PubMed]
33. Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–8.[PubMed]
34. Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–9.[PubMed]
35. Ford CP, Beckstead MJ, Williams JT. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J Neurophysiol. 2007;97(1):883–91.[PubMed]
36. Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain Res. 1995;683(1):25–35.[PubMed]
37. Nylander I, Hyytia P, Forsander O, Terenius L. Differences between alcohol-preferring (AA) and alcohol-avoiding (ANA) rats in the prodynorphin and proenkephalin systems. Alcohol Clin Exp Res. 1994;18(5):1272–9.[PubMed]
38. Kreek MJ. Cocaine, dopamine and the endogenous opioid system. J Addict Dis. 1996;15(4):73–96.[PubMed]
39. Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–45.[PubMed]
40. Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir R, Konradi C. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem. 2003;87(4):922–34.[PubMed]
41. Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47 1:242–55.[PubMed]
42. Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem. 1991;57(1):344–7.[PubMed]
43. Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85(6):1604–13.[PubMed]
44. Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology (Berl) 2006;185(4):505–13.[PubMed]
45. Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51(6):811–22.[PubMed]
46. Narita M, Nagumo Y, Miyatake M, Ikegami D, Kurahashi K, Suzuki T. Implication of protein kinase C in the orexin-induced elevation of extracellular dopamine levels and its rewarding effect. Eur J Neurosci. 2007;25(5):1537–45.[PubMed]
47. Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405.[PubMed]
48. Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601.[PubMed]
49. Park ES, Yi SJ, Kim JS, Lee HS, Lee IS, Seong JK, Jin HK, Yoon YS. Changes in orexin-A and neuropeptide Y expression in the hypothalamus of the fasted and high-fat diet fed rats. J Vet Sci. 2004;5(4):295–302.[PubMed]
50. Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1454–65.[PubMed]
51. Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1436–44.[PubMed]
52. Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19(2):376–86.[PubMed]
53. Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–9.[PubMed]