Are you there God Its me dopamine neuron
September 30, 2013 · by Talia Lerner
Dopamine neurons are some of the most studied, most sensationalized neurons out there. Lately, though, they’ve been going through a bit of an identity crisis. What is a dopamine neuron? Some interesting recent twists in dopamine research have definitively debunked the myth that dopamine neurons are all of a kind – and you should question any study that treats them as such.
There are many ways in which a dopamine neuron (defined as a neuron that releases the neurotransmitter dopamine) is not just a dopamine neuron. I’ll focus on three really cool ways here:
- Not all neurons that release dopamine release it in the same place at the same time. Dopamine neurons have different roles in brain function and behavior depending on how they’re hooked up into neural circuits.
- Not all neurons that release dopamine release only dopamine. Some can also release other neurotransmitters, which could have profound effects on how they influence neural circuit function.
- Not all neurons that release dopamine always release dopamine. Some neurons can switch their dopamine synthesis machinery on or off. Because of this ability, they may not even have been recognized in previous research as being dopamine neurons.
Before I describe these exciting new findings, though, let me give you the standard Neuroscience 101 introduction to dopamine neurons. This influential theory of dopamine neuron function comes to us from Wolfram Schultz and colleagues’ 1997 Science paper, “A Neural Substrate of Prediction and Reward.” It showed that dopamine neurons, which fire at some background rate, fire more in response to unpredicted, but not predicted, rewards. Additionally, if you’re expecting a reward and don’t get it, the dopamine neurons fire less. This finding led Schultz et al. to propose that dopamine neurons encode “reward prediction error.” That is, they tell you whether or not things are as good, better, or worse than you expected. Schultz et al. go on to state “The responses of these neurons are relatively homogeneous—different neurons respond in the same manner and different appetitive stimuli elicit similar neuronal responses. All responses occur in the majority of dopamine neurons (55 to 80%).”
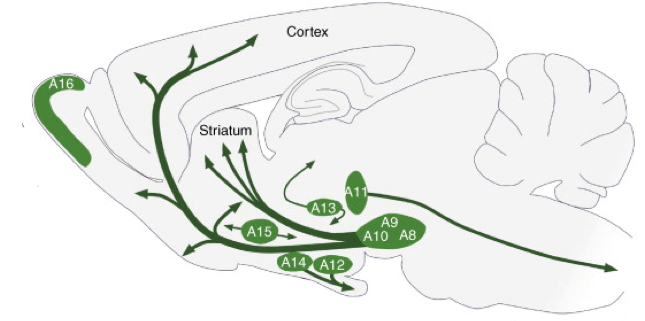
The role of dopamine neurons as computers of reward prediction error remains a fascinating and worthy line of research, but if reward prediction error is ALL that dopamine neurons do, then what do we need 400,000-600,000 of them for?* Here’s a map of where the brain’s dopamine neurons are located (in a cross-section of a rodent brain):
Distribution of dopamine neuron cell groups A8-A16 in the adult rodent brain. Adapted from Björklund, A. & Dunnett, S. B. Dopamine neuron systems in the brain: an update. Trends in Neurosciences 30, 194–202 (2007).
*In humans. There are 160,000-320,000 in monkeys and just 20,000-45,000 in rodents.
Looking at this diagram, there already seem to be some gross anatomical distinctions among groups of dopamine neurons, which is why they are labeled A8-A16. There are also finer anatomical distinctions, which turn out to have not-so-subtle functional implications. In the first line of study I’ll focus on here, Lammel et al. set about distinguishing dopamine neurons in the ventral tegmental area (VTA, or A10 in the above picture) by their connectivity to other brain areas. Lammel et al. observed that there are at least two separable populations of dopamine neurons within the VTA. One population gets input signals from a brain area called the laterodorsal tegmentum and sends output signals to a brain area called the nucleus accumbens (call these LDT-dopamine-NAc neurons). The other population gets inputs from the lateral habenula and sends outputs to the prefrontal cortex (call these LHb-dopamine-PFC neurons). So what? Does the fact that these dopamine neurons are wired into different brain circuits matter at all for behavior? Lammel et al. showed that it does matter. When (using optogenetics!) they activated the inputs to LDT-dopamine-NAc neurons in mice, they found that the animals formed positive associations with the context in which they were stimulated. They chose to spend more time in the part of a box where they’d gotten the brain stimulation. In contrast, when Lammel et al. activated the inputs to LHb-dopamine-PFC neurons, the exact opposite was observed. Animals avoided a part of a box where they’d gotten the stimulation. In another study by the same group, when the mice naturally experienced something good or something bad, the strengths of these distinct circuits were modulated differentially. Mice given cocaine showed an increased strength of the LDT-dopamine-NAc pathway, but no change in the LHb-dopamine-PFC pathway. Mice given an irritant on their paw showed no change in the LDT-dopamine-NAc pathway, but an increased strength of the LHb-dopamine-PFC pathway.
Undermining Schultz et al.’s initial assertion that dopamine neurons are homogenous, Lammel et al. discovered that they are not. This revision likely occurred because of the increasing sensitivity of the tools available, which changed quite a bit from the 1990s to the 2010s. Newer and better tools, in combination with a little creativity, allowed Lammel et al. to distinguish subtleties that weren’t accessible to Schultz et al. In revealing these subtleties, Lammel et al. helped to demonstrate the hubris of believing that you’ve figured out a whole class of neurons because you see responses in 55-80% of a population, especially when you’re not entirely sure (or shouldn’t be) about the criteria you’ve used to define that population. (The question of defining dopamine neurons during in vivo neural recordings is a WHOLE other issue). All the credit in the world to Schultz et al. for lighting the fire of dopamine research, but it was more of a starting point than an end point.
Grouping neurons by the brain circuits they participate in makes a ton of sense if you’re trying to figure out how brain circuits work. But what if you’re trying to figure out the dopamine part of dopamine neurons? Most dopamine neuron research has assumed that when a dopamine neuron fires, it releases the neurotransmitter dopamine, a small molecule that looks like this:
In fact, that’s how we defined “dopamine neuron.” However, as is often the case in science, the situation turns out not to be so simple. In the second line of recent research I’ll discuss here, scientists showed evidence that dopamine neurons can co-release other neurotransmitter molecules, called glutamate and GABA, along with dopamine.
Actually, different subsets of dopamine neurons most likely predominantly co-release either glutamate or GABA. Studies by Hnasko et al. and Stuber et al. demonstrated that dopamine neurons in the VTA co-release glutamate. First, they noticed that many VTA dopamine neurons express a glutamate transporter called VGLUT2, a protein that packages glutamate for release from neurons. Did the presence of VGLUT2 mean that dopamine neurons were packaging glutamate in addition to dopamine? To look at this question, the scientists looked at the responses of neurons in the nucleus accumbens (one place that dopamine neurons send outputs to, see the discussion of Lammel et al. above) to dopamine neuron stimulation. Indeed, they observed fast, excitatory responses of nucleus accumbens neurons to stimulation of VTA dopamine neurons of a type that would be consistent with a glutamatergic rather than a dopaminergic response. These responses were blocked by antagonists of glutamate receptors but not by antagonists of dopamine receptors. Additionally, in mice genetically manipulated to lack VGLUT2 in dopamine neurons, no such responses were seen.
The co-release of glutamate may not occur in all dopamine neurons though. As in Lammel et al.’s studies, connectivity matters. Stuber et al. observed that dopamine neurons in a neighboring area called the substantia nigra (A9), which sends outputs to the dorsal striatum, did not display evidence of glutamate release. That negative result is still controversial. Another group, Tritsch et al., did observe some evidence of glutamate release by substantia nigra dopamine neurons. Additionally, they demonstrated that these substantia nigra dopamine neurons also co-release yet another neurotransmitter: GABA. Oddly, however, substantia nigra dopamine neurons don’t express VGAT, the normal GABA transporter. Instead, Tritsch et al. found that VMAT, the dopamine transporter, can also co-transport GABA, packaging it for synaptic release along with dopamine. Tritsch et al.’s finding might generalize beyond substantia nigra dopamine neurons. As long as there’s some GABA around, anything expressing VMAT could potentially package and release that GABA as well. One key question that arises from Tritsch et al.’s study is exactly where and when the GABA in the substantia nigra is being synthesized. Nevertheless, it’s there.
The implications of glutamate and GABA co-release from dopamine neurons for the most part remain to be seen. The only reported behavioral effect is from the Hnasko et al. paper. They show that mice lacking VGLUT2 in dopamine neurons run around less in responses to cocaine than normal mice. That’s it for now. If nothing else, it demonstrates how much more we have to learn about the phenomenon of transmitter co-release.
So far, we’ve seen that dopamine neurons can signal different things if they’re hooked up into different brain circuits, and that they can play their assigned role in a brain circuit at least in part using chemicals other than just dopamine. In the third line of research I’ll examine here, we’ll add yet another layer of really cool complexity to the picture: dopamine neurons can change the way that they participate in a brain circuit by changing whether or not they’re making and releasing dopamine at all. In this case, Dulcis et al. looked at a slightly different group of dopamine neurons from the ones I’ve been talking about until now, located in the hypothalamus. They noticed that the number of dopamine neurons in rats seemed to fluctuate with the length of “daylight” experienced by the rats. I put daylight in quotes because it’s not real daylight – just whether or not the lights are on in a very controlled laboratory setting. Most lab animals see 12 hours of light per day, but Dulcis et al. also tried just 5 hours per day or up to 19. Rats that experienced long days had fewer dopamine neurons in their hypothalamus, while rats that experienced short days had more. Upon further examination, they determined that the changes in the number of dopamine neurons in the different light conditions weren’t due to neurons dying and being born. The same neurons were actually there in all conditions, but they were switching their dopamine-ness on or off. It’s still unclear why light exposure causes these changes, or what the exact behavioral consequences are. Rats that had long days, and fewer dopamine neurons as a result, displayed depressive and anxious behaviors (keep in mind that rats are nocturnal and prefer the dark). So did rats whose hypothalamic dopamine neurons were killed with a toxin. However, if the dopamine neurons were killed with a toxin while the rats got 12 hours of light per day and then the rats were given only 5 hours of light per day, previously non-dopaminergic neurons were recruited to release dopamine and fewer depressive and anxious behaviors were observed. Pretty cool! And importantly, this work demonstrates that neurons we wouldn’t even have previously identified as dopamine neurons can transform under the right conditions. Some aspects of our brains are built to be stable, but many are changing all the time, allowing us to internalize and adapt to our experience.
After all these studies, what have we learned? To me, the big picture takeaway is that understanding the brain means appreciating complexity. To be slightly more specific, it means linking molecules and cells with circuits and behavior to provide definitions of biological entities that span modalities of study. No more grouping neurons solely by one neurotransmitter they can release. That grouping could sometimes still be relevant, but as we’ve seen in the above studies, not always. Thinking about redefining the group formally known as dopamine neurons, we also need to take a look back at the decades of previous literature with the perspective that hindsight provides. It’s not that the data in older dopamine neuron studies are wrong, but conclusions may not be quite what we thought they were. Which could be a good thing. Many arcane arguments about exactly what dopamine neurons encode may actually end up being settled by understanding that they do many different things in different contexts. Don’t be alarmed: it may seem confusing, but this is the very normal process of science maturing. Not only is the process normal, it is absolutely crucial. Scientists must constantly question and revise our definitions to reflect significant conceptual advances.
Definitions can be confusing. They can also be rather boring, and I worry that they too often drive people away from science. When I was a beginning biology student, I spent hours upon hours making flashcards to help myself memorize what seemed like endless definitions. I viewed it as a tedious but necessary initiation to the biologists’ club. Basically, although it kind of stunk, I told myself that I had to learn the vocabulary to be able to discuss higher order issues with working scientists. What I’ve come to appreciate as I’ve progressed further in my career is how nuanced those once seemingly black-and-white, right-or-wrong definitions are – how much subtlety and history is packed into them. Scientific definitions, like the definition of a dopamine neuron, don’t just provide a common language; they structure the very nature of our investigations. We require this structure in order to proceed with our experiments, but, as we do so, we also need to be aware of the ways in which these definitions can limit us. We compare defined groups to each other. We talk about group averages. So exactly which things are included in our groups can dramatically affect how our data look and what we decide they mean. Thus, we must always be aware of the biases inherent in our categorizations. Perhaps definitions are not so boring after all! Discussions of these caveats could spice up intro course material quite a bit while teaching students how to think like real scientists.
The particular question of defining neuronal cell types actually turns out to be fairly timely. Just a couple weeks ago, the first interim report from the BRAIN initiative working group came out (see also Astra Bryant’s post on the topic). In it, nine high priority research areas for FY 2014 are outlined, the first of these being “generate a census of cell types.” The report recognizes the issues I’ve been discussing here:
There is not yet a consensus on what a neuronal type is, since a variety of factors including experience, connectivity and neuromodulators can diversify the molecular, electrical and structural properties of initially similar neurons. In some cases, there may not even be sharp boundaries separating subtypes from each other. Nonetheless, there is general agreement that types can be defined provisionally by invariant and generally intrinsic properties, and that this classification can provide a good starting point for a census. Thus, the census should begin with well-described large classes of neurons (e.g. excitatory pyramidal neurons of the cortex) and then proceed to finer categories within these classifications. This census would be taken with the knowledge that it will initially be incomplete, and will improve over iterations.
The answer to the question “What is a dopamine neuron?” isn’t quite forthcoming, but high-profile recognition of the question, and the funding that should follow it, is an important first step. Cheers to that.