Neurosci Biobehav Rev. 2011 Jan;35(3):939-55. doi: 10.1016/j.neubiorev.2010.10.014. Epub 2010 Nov 2.
- 1Bernard B. Brodie Department of Neuroscience, University of Cagliari, Cittadella Universitaria, 09042 Monserrato, CA, Italy. mrmelis@unica.it
A B S T R A C T
Oxytocin is a potent inducer of penile erection when injected into the central nervous system. In male rats, the most sensitive brain area for the pro-erectile effect of oxytocin is the paraventricular nucleus of the hypothalamus. This nucleus and surrounding regions contain the cell bodies of all oxytocinergic neurons projecting to extra-hypothalamic brain areas and the spinal cord. This review shows that oxytocin induces penile erection also when injected in some of these areas (e.g., ventral tegmental area, ventral subiculum of the hippocampus, posteromedial cortical nucleus of the amygdala and thoraco-lumbar spinal cord). Microinjection studies combined with intra-cerebral microdialysis and double immunofluorescence studies suggest that oxytocin in these areas activates directly or indirectly (mainly through glutamic acid) mesolimbic dopaminergic neurons. Dopamine released in the nucleus accumbens in turn activates neural pathways leading to the activation of incerto-hypothalamic dopaminergic neurons in the paraventricular nucleus. This activates not only oxytocinergic neurons projecting to the spinal cord and mediating penile erection, but also those projecting to the above extra-hypothalamic areas, modulating directly or indirectly (through glutamic acid) the activity of mesolimbic dopaminergic neurons controlling motivation and reward. Together these neural pathways may constitute a complex hypothetical circuit, which plays a role not only in the consummatory phase of sexual activity (erectile function and copulation), but also in the motivational and rewarding aspects of the anticipatory phase of sexual behaviour.
1.Introduction
Penile erection is a male sexual response that plays a key role in reproduction of mammals including man, and that can be also observed in contexts different from those strictly related to reproduction. Depending on the context in which penile erection occurs, different central and peripheral neural and/or humoral mechanisms participate in its regulation (see Meisel and Sachs, 1994; Argiolas and Melis, 1995, 2004, 2005; Sachs, 2000, 2007; McKenna, 2000; Giuliano and Rampin, 2000, 2004; Andersson, 2001; Melis and Argiolas, 1995a, 2003; Hull et al., 2002). Among central neurotransmitters and neuropeptides that control penile erection, the best known are dopamine, serotonin, excitatory amino acids, nitric oxide, adrenocorticotropin, oxytocin and opioid peptides. They can facilitate or inhibit penile erection by acting in several brain areas, i.e., the medial preoptic area, the paraventricular nucleus of the hypothalamus, the ventral tegmental area, the hippocampus, the amygdala, the bed nucleus of the stria terminalis, the nucleus accumbens, the medulla oblongata and the spinal cord (Table 1) (see Meisel and Sachs, 1994; Witt and Insel, 1994; Stancampiano et al., 1994; Argiolas and Melis, 1995, 2005; Argiolas, 1999; Bancila et al., 2002; Giuliano and Rampin, 2000, McKenna, 2000; Andersson, 2001; Hull et al., 2002; Coolen et al., 2004).
Oxytocin, the neurohypophyseal peptide well known for its hormonal role in lactation and parturition, is present in females and males, not only in neurons with cell bodies located in the paraventricular and supraoptic nuclei of the hypothalamus projecting to the neurohypophysis, but also in neurons projecting from the paraventricular nucleus and surrounding structures to extrahypothalamic brain areas (i.e., the septum, the ventral tegmental area, the hippocampus, the amygdala, the medulla oblongata and the spinal cord). These neurons are thought to be involved in numerous central functions, such as memory, learning, affiliative and socio-sexual behaviours, including penile erection and copulatory behaviour (see Buijs, 1978; Sofroniew, 1983; Argiolas and Gessa, 1991; Pedersen et al., 1992; Carter, 1992; Wagner and Clemens, 1993; Ivell and Russel, 1995; Carter et al., 1997; Tang et al., 1998; Veronneau-Longueville et al., 1999). Indeed, oxytocin facilitates erectile function and male sexual behaviour in mice, rats, rabbits and monkeys (see Argiolas and Gessa, 1991; Carter, 1992; Pedersen et al., 1992; Argiolas and Melis, 1995, 2004; Argiolas, 1999). This may occur also in humans, since plasma oxytocin is increased by sexual stimuli, especially at ejaculation (Carmichael et al., 1987; Murphy et al., 1987) and by the manipulation of breast and of the genitalia, which usually occur during sexual intercourse (Tindall, 1974).
A facilitative effect of oxytocin on male sexual behaviour was first demonstrated by the ability of intravenous oxytocin to decrease the latency to the first ejaculation and to retard sexual exhaustion of male rabbits paired with receptive females (Melin and Kihlstrom, 1963). However, the sexual effects of oxytocin were definitively recognized only in the eighties. Oxytocin given centrally in nanogram amounts was then found able to induce penile erection (Argiolas et al., 1985, 1986) and to improve copulatory behaviour (Arletti et al., 1985) in male rats, and to increase lordosis in female rats (Arletti and Bertolini, 1985; Caldwell et al., 1986), apparently by acting on uterine-type oxytocinergic receptors (see Argiolas and Melis, 1995, 2004; Argiolas, 1999; Melis and Argiolas, 2003; and references therein). Oxytocin improves sexual behaviour not only in sexually potent male rats (Arletti et al., 1985) but also in aged male rats (Arletti et al., 1990), and in dominant, but not in subordinate, male squirrel monkeys (Winslow and Insel, 1991).
The pro-erectile effect of oxytocin is testosterone-dependent, since it is abolished by hypophysectomy and castration, and restored by supplementation with testosterone or its metabolites, estradiol and 5_-dihydro-testosterone given together (Melis et al., 1994a). The most sensitive brain area for the induction of penile erection by oxytocin is the paraventricular nucleus of the hypothalamus (Melis et al., 1986), from which all extra-hypothalamic oxytocinergic projections originate (see above). Here, oxytocin was found to be able to induce penile erection (and yawning) when injected at doses as low as 3 pmol (see Section 2.1 below). Oxytocin induced penile erection also when injected bilaterally into the CA1 field of the hippocampus, but not in the dorsal subiculum (see Section 2.3 below), the lateral septum, the caudate nucleus, the medial preoptic area, the ventromedial nucleus of the hypothalamus and the supraoptic nucleus (Melis et al., 1986). As to the mechanism by which oxytocin acts in the paraventricular nucleus to induce this sexual response, numerous studies suggest that oxytocin activates its own neurons. In line with this hypothesis, sexual interaction increases FOS, the gene product of the immediate early gene c-fos in paraventricular oxytocinergic neurons projecting to the spinal cord, which are involved in the control of penile erection (see Witt and Insel, 1994 and references therein), and sexual impotence (e.g., the inability of an adult male rat to copulate with an ovariectomized oestrogen-progesteroneprimed receptive female) has been associated in the male rat with low levels of oxytocin mRNA in the paraventricular nucleus of the hypothalamus (Arletti et al., 1997).
Whether oxytocin influences the anticipatory phase or the consummatory phase of sexual behaviour is unclear at present. As oxytocin induces penile erection and the main effect of oxytocin on copulatory behaviour is a decrease in the post-ejaculatory interval in male rats (Arletti et al., 1985), it is reasonable to assume that the peptide improves sexual performance. However, as oxytocin also increases socio-sexual interaction (see Pedersen et al., 1992; Carter et al., 1997; Ivell and Russel, 1995), and oxytocin receptor antagonists prevent noncontact erections (Melis et al., 1999a), which are considered as an index of sexual arousal (see Sachs, 1997, 2000, 2007; Melis et al., 1998, 1999b and references therein), a possible role of oxytocin in sexual arousal and sexual motivation cannot be ruled out.
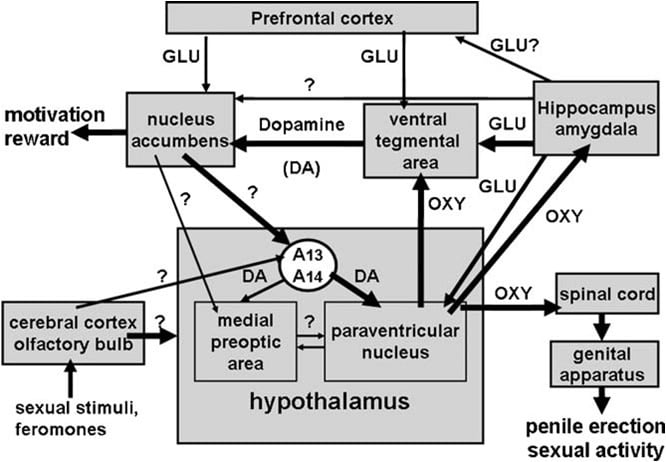
This review summarizes published and unpublished results of recent studies, which show that oxytocin induces penile erection not only when injected into the paraventricular nucleus of the hypothalamus, but also in other extra-hypothalamic brain areas, such as the ventral tegmental area (Melis et al., 2007, 2009a; Succu et al., 2008), the ventral subiculum of the hippocampus and the posterior nucleus of the amygdala (Melis et al., 2009b, 2010), which are important constituents of the limbic system and are thought to play a key role in motivation and reward processes. These studies reveal that oxytocin participates in neural circuits, which include other neurotransmitters, such as dopamine and glutamic acid, and other brain areas other than the paraventricular nucleus, e.g., the ventral tegmental area, the nucleus accumbens, the hippocampus and areas yet to be identified. These circuits are likely to mediate an interaction between the mesolimbic and the incerto-hypothalamic dopaminergic system, and to play a role not only in the consummatory phase of male sexual behaviour (e.g., penile erection and copulation), but also in sexual motivation and sexual arousal, hence providing a neural substrate for explaining the motivational and rewarding properties of sexual activity.
2. Oxytocin influences penile erection by acting in different brain areas
2.1. The paraventricular nucleus of the hypothalamus
As recalled above the paraventricular nucleus of the hypothalamus was soon identified as the brain area most sensitive for the pro-erectile effect of oxytocin. When injected unilaterally in this nucleus, oxytocin was found active at doses as low as 3 ng (3 pmol) (Melis et al., 1986). Structure-activity relationship studies revealed that oxytocin-induced penile erection was mediated by uterinetype oxytocin receptors, coupled to a Ca2+ influx into the cell bodies of oxytocinergic neurons projecting to extra-hypothalamic brain areas and to the activation of nitric oxide-synthase. Nitric oxide in turn by acting as an intracellular messenger with a yet unknown mechanism (not involving guanylate cyclase) leads to the activation of oxytocinergic neurons projecting to the spinal cord and extra-hypothalamic brain areas, inducing penile erection (Fig. 1) (see below and Argiolas and Melis, 1995, 2004, 2005 and references therein). The ability of oxytocin to activate its own neurons
Fig. 1. (MISSING) Schematic representation of oxytocinergic neurons, which originate in the paraventricular nucleus of the hypothalamus and project to extra-hypothalamic brain areas, such as the spinal cord, the VTA, the hippocampus, the amygdala, etc. The activation of these neurons by dopamine, excitatory amino acids, oxytocin itself, hexarelin analogue peptides and VGF-derived peptides leads to penile erection, which can be reduced and/or abolished by the stimulation of GABAergic, opioid and cannabinoid CB1 receptors. The activation of oxytocinergic neurons is secondary to the activation of nitric oxide-synthase present in these neurons. Indeed endogenous nitric oxide formed by the stimulation of dopamine, excitatory amino acid or oxytocin receptors or exogenous nitric oxide, as that derived from nitric oxide donors given directly into the paraventricular nucleus, activates oxytocinergic neurons by a yet unidentified mechanism, apparently not related to the stimulation of guanylate cyclase. This causes in turn the release of oxytocin in the spinal cord and in extra-hypothalamic brain areas. Some details on the mechanisms by which oxytocin induces penile erection when released in these areas, e.g., the VTA, the ventral subiculum and the amygdala are described in the respective brain area sections. Here, oxytocin acts on its own receptors and increases NO production, which leads to penile erection as found in the PVN. However, at variance with the PVN, in the caudal VTA NO activates guanylate cyclase. This causes an increase in cGMP concentration leading to the activation of mesolimbic dopaminergic neurons and to penile erection. In the VS NO activates glutamatergic neurons projecting to extra-hippocampal areas, including the VTA. Glutamic acid in the VTA activates in turn mesolimbic dopaminergic neurons as found with oxytocin. Mechanisms similar to those described above are likely to operate also when penile erection occurs in physiological contexts, namely as when male rats are placed in the presence of an inaccessible receptive female (e.g., noncontact erections) or during copulation.
in the paraventricular nucleus was supported by studies showing that: (1) oxytocin receptors are present in this hypothalamic nucleus (Freund-Mercier et al., 1987; Freund-Mercier and Stoeckel, 1995); (2) oxytocin facilitates its own release in vitro and in vivo (Freund-Mercier and Richard, 1981, 1984; Moos et al., 1984); and (3) oxytocin excites its own neurons by acting in the paraventricular nucleus (Yamashita et al., 1987). Moreover, oxytocinergic synapses impinging on the cell bodies of magnocellular oxytocinergic neurons have also been identified in the paraventricular and supraoptic nucleus of the hypothalamus (Theodosis, 1985). Finally, destruction of central oxytocinergic neurons by electrolytic or chemical excitotoxic lesions of the paraventricular nucleus, which completely depletes oxytocin content across the central nervous system and the spinal cord, abolishes not only the pro-erectile effect of oxytocin, but also impairs drug-induced penile erection and noncontact erections (see below and Argiolas et al., 1987a,b; Liu et al., 1997 and references therein). Results similar to those found with lesions of the paraventricular nucleus are found with potent and selective oxytocin receptor antagonists. Indeed, these compounds injected into the paraventricular nucleus in nanogram amounts prevented completely oxytocin-induced penile erection, while when given into the lateral ventricles prevented not only penile erections induced by oxytocin itself, but also drug-induced penile erection (see the Section 3 below and Argiolas and Melis, 1995, 2004, 2005 and references therein) and noncontact erections (Melis et al., 1999a), and were moreover extremely effective in impairing copulatory behaviour of sexually potent male rats (Argiolas et al., 1988). Furthermore, sexual interaction increases FOS, the gene product of the immediate early gene c-fos in paraventricular oxytocinergic neurons projecting to the spinal cord involved in the control of penile erection (see Witt and Insel, 1994 and references therein). Finally, sexual impotence (e.g., the inability of an adult male rat to copulate with an oestrogen-progesteroneprimed receptive female) has also been associated in the male rat with low levels of oxytocin mRNA and of nitric-oxide synthase in the paraventricular nucleus of the hypothalamus (Benelli et al., 1995; Arletti et al., 1997) (for an extensive review of these studies see Argiolas, 1999; Argiolas and Melis, 2004, 2005).
2.2. The ventral tegmental area
The ventral tegmental area was discovered only recently as a brain site in which oxytocin induces penile erection. This area contains oxytocinergic nerve endings originating in the paraventricular nucleus and oxytocin receptors (Freund-Mercier et al., 1987; Vaccari et al., 1998). More precisely, oxytocin was found capable of inducing penile erection when injected unilaterally into the caudal, but not in the rostral ventral tegmental area in a dose-dependent manner (Melis et al., 2007). The active doses were higher than those required when injected into the paraventricular nucleus and similar to those inducing penile erection when injected into the ventral subiculum of the hippocampus or into the posteromedial cortical nucleus of the amygdala (see below). Apparently, the pro-erectile effect is mediated by the activation of mesolimbic dopaminergic neurons projecting to the shell of the nucleus accumbens, which in turn activates yet unknown neural pathways projecting to the incerto-hypothalamic dopaminergic neurons impinging on paraventricular oxytocinergic neurons mediating penile erection (Melis et al., 2007, 2009a).
As to the mechanism by which oxytocin activates dopaminergic neurotransmission in the ventral tegmental area, the available data suggest that oxytocin stimulates oxytocinergic receptors located in the cell bodies of mesolimbic dopaminergic neurons. This increases Ca2+ influx inside the cell bodies of dopaminergic neurons, thereby activating nitric oxide-synthase (Succu et al., 2008). At variance with the paraventricular nucleus (see the Section 3 below), nitric oxide in turn activates guanylate cyclase, hence increasing the concentration of cyclic GMP. In line with this mechanism, either d(CH2)5Tyr(Me)2-Orn8-vasotocin, a potent oxytocin antagonist, or S-methyl-thio-l-citrulline, a potent inhibitor of neuronal nitric oxide-synthase, injected into the caudal ventral tegmental area before oxytocin, abolished penile erection and the increase in extra-cellular dopamine concentration in the shell of the nucleus accumbens induced by oxytocin. Moreover, 8-bromo-cyclic GMP, an active phosphodiesterase-resistant cyclic GMP analogue, induces penile erection when injected into the caudal ventral tegmental area and increases extra-cellular dopamine concentration in the shell of the nucleus accumbens, as found with oxytocin injected into the caudal ventral tegmental area (Succu et al., 2008; Melis et al., 2009a) (see also Fig. 2).
Always in line with this mechanism, haloperidol, a potent dopamine D2 receptor antagonist, injected into the shell of the nucleus accumbens reduces penile erection induced by oxytocin injected into the ventral tegmental area (Melis et al., 2007). The above mechanism is also supported by double immuno-fluorescence studies, showing that oxytocin fibres impinge on the cell bodies of dopaminergic neurons in the caudal ventral tegmental area, which were previously labeled with the retrograde tracer Fluorogold injected into the shell of the nucleus accumbens (Melis et al., 2007; Succu et al., 2008). The activations of these dopaminergic neurons and of dopamine receptors in the nucleus accumbens lead in turn to the activation of neural pathways yet to be identified, which stimulate incerto-hypothalamic dopaminergic neurons to release dopamine in the paraventricular nucleus, thereby activating oxytocinergic neurons projecting to the spinal cord and mediating penile erection (see above and Melis et al., 2007; Succu et al., 2007, 2008). Indeed, oxytocin injected into the caudal ventral tegmental area at a dose that induced penile erection, increased extra-cellular dopamine concentration in the dialysate obtained not only from the nucleus accumbens, but also from the paraventricular nucleus (Succu et al., 2007).
2.3. The hippocampus
The CA1 field of the hippocampus was the other brain area rich in oxytocinergic fibres and receptors identified by the earlier studies in which the injection of oxytocin induced penile erection (see Bujis, 1978; Sofroniew, 1983). However, at variance with the paraventricular nucleus, here oxytocin was found capable of inducing penile erection only when injected bilaterally and at higher doses than those found active in the paraventricular nucleus (Melis et al., 1986; Chen et al., 1992). Injections of oxytocin into the subiculum were found inactive in these earlier studies. However, recent and more careful microinjection studies lead to the identification of a region of the ventral subiculum in which the injection of oxytocin was capable of inducing penile erection in a dose-dependent manner (Melis et al., 2009b). The pro-erectile effect of oxytocin injected into this brain area was observed at doses similar to those found active in the ventral tegmental area after unilateral injection (Melis et al., 2007), as found in the paraventricular nucleus. Apparently, oxytocin injected into the ventral subiculum induces penile erection by activating oxytocinergic receptors in neurons containing nitric oxide-synthase, causing an increase in nitric oxide production. Nitric oxide in turn by acting as intercellular messenger activates glutamic acid neurotransmission, leading to penile erection, possibly through neural (glutamatergic) efferent projections from the ventral subiculum to extra-hippocampal brain areas modulating the activity of mesolimbic dopaminergic neurons (e.g., the ventral tegmental area, the prefrontal cortex, the paraventricular nucleus) (see below and Melis, 2007, 2009b; Succu et al., 2008).
This mechanism of action is supported by intra-cerebral microdialysis experiments, which show that oxytocin injected into the ventral subiculum at doses that induce penile erection, increases nitric oxide production and extracellular glutamic acid concentration in the dialysate from the ventral subiculum (Melis et al., 2010) and of extracellular dopamine in the nucleus accumbens (Melis et al., 2007). These responses were antagonized not only by the oxytocin receptor antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin, but also by the neuronal nitric oxide-synthase inhibitor S-methyl-thio-lcitrulline and by the nitric oxide scavenger haemoglobin given into the ventral subiculum few minutes before oxytocin (Melis et al., 2010).
Moreover, in line with this mechanism of action, activation of glutamatergic neurotransmission by NMDA injected into the ventral subiculum induces penile erection (Melis et al., 2010). The phenotype of efferent projections from the ventral subiculum, which cause the activation of mesolimbic dopaminergic neurons and the increase of extra-cellular dopamine in the nucleus accumbens, is unknown at present. However, since penile erection induced by oxytocin injected into the ventral subiculum occurs concomitantly to an increase of extra-cellular glutamic acid in the dialysate from the ventral tegmental area, but not from the nucleus accumbens and is antagonized by (+)MK-801, a potent non-competitive antagonist of excitatory amino acid receptors of the NMDA subtype (Woodruff et al., 1987), injected into the ventral tegmental area, but not in the nucleus accumbens (see Fig. 2 and Melis et al., 2009b), it is likely that these projections lead to the activation of glutamatergic neurotransmission in the ventral tegmental area, which in turn activates mesolimbic dopaminergic neurons projecting to the nucleus accumbens. Whether the increased concentration of glutamic acid found in the ventral tegmental area after oxytocin injection into the ventral subiculum is released from neurons originating in the subiculum or in other brain areas (e.g., the prefrontal cortex) is unknown at present. Nonetheless, this causes the activation of mesolimbic dopaminergic neurons and an increased release of dopamine in the nucleus accumbens. Here the activation of dopamine receptors leads to the activation of incerto-hypothalamic dopaminergic neurons, releasing dopamine in the paraventricular nucleus, thereby activating oxytocinergic neurons projecting to the spinal cord and mediating penile erection (see above and Melis et al., 2007, 2009a; Succu et al., 2008).
2.4. The amygdala
The amygdala is another brain area rich in oxytocin fibres and receptors (see Freund-Mercier et al., 1987; Vaccari et al., 1998; Uhl-Bronner et al., 2005). Oxytocin here is thought to be involved in different functions, from anxiolysis, social memory and cognition, socially reinforced learning, emotional empathy, emotional face processing and fear in humans to erectile function and sexual behaviour (see Kondo et al., 1998; Dominguez et al., 2001; Ebner et al., 2005; Huber et al., 2005; Domes et al., 2007; Petrovic et al., 2008; Lee et al., 2009; Donaldson and Young, 2009; Hurlemann et al., 2010). However, the ability of oxytocin to induce penile erection in male rats when induced in the posteromedial cortical nucleus of the amygdala was discovered only recently (Melis et al., 2009b). This response occurred concomitantly with an increase in extra-cellular dopamine concentration in the dialysate obtained from the shell of the nucleus accumbens, as found after oxytocin injection into the ventral subiculum (Melis et al., 2009b). The mechanism by which oxytocin injected into the posteromedial cortical nucleus of the amygdala induces penile erection is unknown at the moment. The available data show that both penile erection and the increase in extra-cellular dopamine concentration in the dialysate obtained from the nucleus accumbens are mediated by the activation of oxytocinergic receptors, as both responses were abolished by the oxytocin receptor antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin injected in the amygdala nucleus few minutes before oxytocin (Melis et al., 2009b).
Whatever mechanism oxytocin activates in the posteromedial cortical nucleus of the amygdala, the sexual response induced by the peptide is abolished by the blockade of all dopaminergic receptors with cis-flupenthixol injected into the shell of the nucleus accumbens and by the blockade of NMDA receptors with (+)MK-801 injected into the ventral tegmental area, but not into the nucleus accumbens, as found for penile erection induced by oxytocin injected into the ventral subiculum (Melis et al., 2009b). This suggests that oxytocin injected into the posteromedial nucleus of the amygdala activates glutamic acid neurotransmission in the ventral tegmental area. This causes in turn the activation of mesolimbic dopaminergic neurons, leading to penile erection. In view of studies showing neural pathways interconnecting this nucleus of the amygdala with the ventral subiculum (Canteras et al., 1995; French and Totterdell, 2003), these findings raise the possibility that an interaction may exist between these two brain areas, although direct pathways from the amygdala either to the nucleus accumbens or to the ventral tegmental area have been described (Kelley and Domesick, 1982; Witter, 2006).
2.5. The spinal cord
The spinal cord is another area of the central nervous system that contains oxytocinergic fibres and receptors (Freund-Mercier et al., 1987; Uhl-Bronner et al., 2005), in which oxytocin induces penile erection (Tang et al., 1998; Veronneau-Longueville et al., 1999; Giuliano and Rampin, 2000; Giuliano et al., 2001). As recalled above, these oxytocinergic fibres originate in the paraventricular nucleus of the hypothalamus and contribute to descending pathways controlling spinal autonomic neurons mediating penile erection. Indeed these fibres make synaptic contacts in the dorsal horn preganglionic sympathetic and parasympathetic cell columns in the thoraco-lumbar and lumbo-sacral tract with spinal neurons innervating penile cavernous corpora (Marson and McKenna, 1996; Giuliano and Rampin, 2000; Giuliano et al., 2001). These synaptic contacts were demonstrated by labeling of spinal neurons originating in the penis and reaching the spinal cord with specific retrograde tracers injected into cavernous corpora, combined with double immuno-fluorescence and confocal laser microscopy studies (Tang et al., 1998; Veronneau-Longueville et al., 1999). In line with these studies, in anaesthetized male rats intrathecal injection of cumulative doses of oxytocin at the lumbo-sacral, but not at the thoraco-lumbar level, elicited intracavernous pressure rises in a dose dependent manner. These effects were abolished by the blockade of oxytocinergic receptor with d(CH2)5Tyr(Me)2-Orn8-vasotocin and by section of the pelvic nerves (Giuliano and Rampin, 2000; Giuliano et al., 2001). These results demonstrate that oxytocin, acting at the lumbo-sacral spinal cord, increases intracavernous pressure, and suggest that oxytocin, released during physiological activation of the paraventricular nucleus is a potent activator of spinal pro-erectile neurons projecting to the cavernous corpora. Interestingly, these pro-erectile spinal neurons on which oxytocin acts to exert its pro-erectile effect, also receive synaptic contacts from serotoninergic neurons originating in the nucleus paragigantocellularis of the reticular formation of the medulla oblongata (Marson and McKenna, 1992; Tang et al., 1998). Destruction of these serotoninergic neurons facilitates ejaculation and penile reflexes in male rats (Marson and McKenna, 1992; Yells et al., 1992). Since drugs that stimulate 5HT2C receptors facilitate penile erection when given intracerebroventricularly, but not into the paraventricular nucleus, and drugs that that block 5HT2C receptors reduce also dopamine agonistand oxytocin-induced penile erection, while dopamine antagonists do not reduce 5HT2C agonist-induced penile erection (see Stancampiano et al., 1994 and references therein), it has been also suggested that oxytocin facilitates the action of pro-erectile 5HT2C receptors at the level of the lumbo-sacral spinal cord (Stancampiano et al., 1994). Alternatively, oxytocin might influence the activity of spinal descending serotoninergic neurons by acting directly in the nucleus paragigantocellularis, where these neurons originate (see Stancampiano et al., 1994). In line with this possibility, an oxytocinergic pathway originating in the paraventricular nucleus and reaching the nucleus paragigantocellularis of the reticular formation in the medulla oblongata has been described (Bancila et al., 2002), and the activation of 5HT2C receptors located in the spinal cord down-stream to dopamine and oxytocin receptors is considered a common mechanism of the pro-erectile effect of these compounds and even of ACTH-MSH peptides (Kimura et al., 2008).
3. Interactions between oxytocin, dopamine and glutamic acid in the central nervous system and penile erection
As recalled in Section 1, all oxytocinergic neurons present in the central nervous system originate in the paraventricular nucleus and surrounding structures. The activity of these neurons is under the control of different neurotransmitters and/or neuropeptides. Among the most studied at the paraventricular level are dopamine, glutamic acid, gamma-aminobutyric acid (GABA), nitric oxide, endocannabinoids, opioid peptides, growth hormone-releasing peptides, VGF-related peptides and oxytocin itself. Dopamine, glutamic acid, growth hormone releasing peptides, VGF-derived peptides and oxytocin are stimulatory, e.g., these compounds and their agonists facilitate penile erection when injected into the paraventricular nucleus, while GABA, opioid peptides and endocannabinoids are inhibitory, e.g., these compounds or their agonists inhibit penile erection (see Meisel and Sachs, 1994; Witt and Insel, 1994; Argiolas and Melis, 1995, 2004, 2005; Giuliano and Rampin, 2000, 2004; McKenna, 2000; Andersson, 2001; Hull et al., 2002).
Several lines of experimental evidence suggest that these oxytocinergic neurons and the above neurotransmitters and neuropeptides are involved in the control of erectile function and sexual behaviour in different physiological contexts. Moreover, oxytocin released in extra-hypothalamic brain areas, such as the ventral tegmental area, the hippocampus and its regions, the amygdala and the spinal cord may influence the activity of those neurons on which oxytocinergic synapses impinge. At the moment, the only neurons important for penile erection on which oxytocinergic synapses impinge, identified with certainty, are the cell bodies of mesolimbic dopaminergic neurons of the caudal ventral tegmental area projecting to the shell of the nucleus accumbens (Melis et al., 2007; Succu et al., 2008), and the pro-erectile spinal neurons projecting from the lumbo-sacral tract to the cavernous corpora (see Giuliano and Rampin, 2000; Giuliano et al., 2001) (see also Sections 2.2 and 2.5). Indeed, although oxytocinergic synapses and receptors have been also identified in the ventral subiculum, the amygdala and the spinal cord, areas which are all important for penile erection (see above), in these areas the type of neurotransmitter/s present in the neurons on which oxytocinergic nerve endings impinge, are still unknown.
This section of the review summarizes briefly the recent literature on the mechanisms underlying the pro-erectile effect of oxytocin injected into the caudal ventral tegmental area, the ventral subiculum of the hippocampus and in the spinal cord. Particular attention is given to the interaction of the peptide with dopamine and glutamic acid in these areas and on the role this interaction may play in the central control of erectile function. A brief summary of the effects of dopamine and glutamic acid on oxytocinergic neurons in the paraventricular nucleus, which also play a key role in erectile function is provided first, in order to make the reader aware of the early state of research in this field, as these studies have been already reviewed extensively (see Argiolas and Melis, 1995, 2004, 2005; Melis and Argiolas, 2003). Also in this case, particular attention is given to the most recent results, which suggest an important role for both a dopamine-oxytocin link and a glutamic acid-oxytocin link not only in sexual performance (penile erection and copulation), but also in sexual arousal and sexual motivation.
3.1. Dopamine-oxytocin interaction in the paraventricular nucleus
The ability of dopamine agonists to induce penile erection by activating central oxytocinergic neurons was suggested immediately after the discovery that apomorphine induces penile erection when injected into the paraventricular nucleus (Melis et al., 1987) when the oxytocin receptor antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin injected intracerebroventricularly (i.c.v.) was found able to reduce almost completely penile erection induced not only by oxytocin given i.c.v., but also by apomorphine, given subcutaneously (Argiolas et al., 1987b). These results were followed by those of other studies showing similar results when d(CH2)5Tyr(Me)2-Orn8-vasotocin was given i.c.v. and apomorphine was given directly into the paraventricular nucleus (Melis et al., 1989b), leading to suggest that dopamine agonists induce penile erection by activating paraventricular oxytocinergic neurons projecting to extra-hypothalamic brain areas and in particular to the spinal cord (see Argiolas and Melis, 1995, 2004, 2005). In line with this hypothesis, in anaesthetized rats, blockade of lumbo-sacral oxytocinergic receptors by a non-peptide oxytocin receptor antagonist has been recently found capable of abolishing apomorphine-induced rises in intracavernous pressure induced by the dopamine agonist apomorphine, providing evidence for a paraventriculo-spinal oxytocinergic pathway involved in penile erection (Baskerville et al., 2009).
Studies aimed at the identification of the dopamine receptor responsible for the induction of penile erection, revealed that also in the paraventricular nucleus dopamine receptor agonists induce penile erection by acting on dopamine receptors of the D2 family, as found with dopamine receptor agonists given systemically (see Melis et al., 1987; Eaton et al., 1991; Melis and Argiolas, 1995a). Accordingly, apomorphine, a potent mixed D1/D2 receptor agonist, and quinpirole, a potent selectiveD2receptor agonist, but not SKF 38393, a selective D1 receptor agonist, injected into this hypothalamic nucleus were found able to induce penile erection in a dose-dependent manner, and the sexual response induced by these D2 receptor agonists was abolished by D2 receptor antagonists, such as haloperidol and l-sulpiride, but not by SCH 23390, a selective D1 receptor antagonist (Melis et al., 1987). The ability of apomorphine to induce penile erection when injected into the paraventricular nucleus was also confirmed by telemetry studies showing that the dopamine agonist given into the paraventricular nucleus is able to increase intracavernous pressure in awake male rats without modifying systemic blood pressure (Chen et al., 1999; Giuliano and Allard, 2001), as found after systemic injection (Bernabè et al., 1999). These studies also confirmed a main role of D2 receptors, as D1 receptor agonists were usually found to be unable to increase intracavernous pressure when injected into the paraventricular nucleus (Chen et al., 1999).
Several lines of experimental evidence were then available suggesting that paraventricular D2 receptors, whose stimulation induces penile erection, are located on the cell bodies of oxytocinergic neurons. First, the paraventricular nucleus contains dopaminergic nerve terminals that belong to the so called incertohypothalamic dopaminergic neurons. The cell bodies of these neurons are situated in the A13 and A14 group of Dahlstrom and Fuxe (1964), arborize extensively and innervate several hypothalamic structures, including paraventricular oxytocinergic neurons projecting to the neurohypophysis and/or to extra-hypothalamic brain areas (Buijs et al., 1984; Lindvall et al., 1984).
The involvement of these dopaminergic neurons at the paraventricular level in the control of penile erection and copulation is supported by microdialysis studies showing that the concentrations of extracellular dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC), its main metabolite, are increased in the dialysate obtained from the paraventricular nucleus of sexually potent male rats showing noncontact erections when put in the presence of an inaccessible ovariectomized oestrogen + progesterone-primed receptive female (Melis et al., 2003).
The increase of the dopamine and DOPAC concentrations was even higher when copulation with the receptive female was allowed (Melis et al., 2003), as found in the medial preoptic area (Hull et al., 1995) and in the nucleus accumbens (Pfaus and Everitt, 1995). Second, several studies show that penile erection induced by the stimulation of paraventricular D2 receptors, is mediated by oxytocin released in these areas. Accordingly, apomorphine given at doses that induce penile erection, was found able to increase oxytocin concentration, not only in plasma of rats and monkeys (Melis et al., 1989a; Cameron et al., 1992), but also in extra-hypothalamic brain areas, such as the hippocampus (Melis et al., 1990). In line with these results, apomorphine injected into the paraventricular nucleus at a dose that induces penile erection was recently shown to be able to increase also extra-cellular dopamine concentration in the nucleus accumbens, an effect reduced by the oxytocin receptor antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin injected into the ventral tegmental area (Succu et al., 2007; Melis et al., 2009a) (see also Section 4). Third, bilateral electrolytic lesions of the paraventricular nucleus, which almost completely eliminate oxytocin from extra-hypothalamic brain areas (Hawthorn et al., 1985), abolish apomorphine-induced penile erection (Argiolas et al., 1987a), and selective oxytocin receptor antagonists given into the lateral ventricles, but not into the paraventricular nucleus, reduce dose-dependently apomorphineinduced penile erection with a potency parallel to that of these compounds in blocking oxytocin receptors (Melis et al., 1989b). Oxytocin receptor antagonists are also extremely potent in reducing the facilitation of male sexual behaviour induced not only by oxytocin, but also by apomorphine (Argiolas et al., 1988, 1989).
As to the mechanism by which D2 receptors activated by dopamine or by dopamine receptor agonists, increase the activity of oxytocinergic neurons, thereby releasing oxytocin in extrahypothalamic brain areas and in the spinal cord, numerous experimental data support the hypothesis that the stimulation of D2 receptors increases the concentration of intracellular Ca2+ ions inside the cell bodies of oxytocinergic neurons, leading to the activation of nitric oxide-synthase, a Ca2+-calmodulin-dependent enzyme, which is present in these cell bodies (Vincent and Kimura, 1992; Torres et al., 1993; Sanchez et al., 1994; Sato-Suzuki et al., 1998). The increased nitric oxide production causes in turn the activation of oxytocinergic neurons. Accordingly, (1) apomorphineinduced penile erection was prevented by organic calcium channel blockers and by_-conotoxin GVIA, a potent and selective blocker of voltage-dependent Ca2+ channels of the N-type (McCleskey et al., 1987), given into the paraventricular nucleus (see Argiolas et al., 1990, and references therein); (2) apomorphine-induced penile erection was prevented by nitric oxide-synthase inhibitors given into the paraventricular nucleus (Melis et al., 1994c); and (3) apomorphine and other D2 receptor agonists given at doses that induce penile erection increased nitric oxide production in the paraventricular dialysate obtained by intra-cerebral microdialysis, an increase that was reduced by inhibitors of paraventricular nitric oxide-synthase given at doses that reduce D2 receptor agonistinduced penile erection (Melis et al., 1996). The mechanism by means of which nitric oxide activates paraventricular oxytocinergic neurons, is still unknown, although available data suggest that nitric oxide acts as an intracellular messenger and that guanylate cyclase is not involved. Indeed, the active phosphodiesteraseresistant analogue of cyclic GMP, 8-bromo-cyclic GMP, was found unable to induce penile erection when given into the paraventricular nucleus (Fig. 2) (see Melis and Argiolas, 1995b and references therein).
The above interpretation has been often considered not convincing, mainly because the stimulation of dopamine D2 receptors is usually coupled to inhibition rather than excitation of the cell bodies of the neurons containing these receptors through different G protein coupled mechanisms (see Sokoloff and Schwartz, 1995). However, a possible explanation for this discrepancy, which is in line with a direct stimulation of paraventricular oxytocinergic neurons by dopamine, has been suggested recently by the discovery of a G protein-coupled dopamine D4 receptor, a member of the D2 receptor family (D2, D3 and D4), the stimulation of which increases Ca2+ influx in cell preparations containing a cloned version of this receptor subtype (Moreland et al., 2004). More importantly, a selective D4 receptor agonist (e.g., ABT 724) (N-methyl-4-(2-cyanophenyl)piperazynil-3methylbenzamide maleate) was found capable of inducing penile erection in male rats when given systemically (Brioni et al., 2004). This effect was not found with the selective D2 receptor subtype agonist PNU-95666E (R-5,6-dihydro-N,N-dimethyl-4Himidazo[ 4,5,1-i]quinolin-5-amine) (Hsieh et al., 2004), which was also unable to increase Ca2+ influx in the cell preparations containing the cloned version of the D4 receptor subtype (Brioni et al., 2004; Moreland et al., 2004). In line with the above hypothesis and findings, PD 168,077 (N-methyl-4-(2-cyanophenyl)piperazynil-3methylbenzamide maleate), PIP-3EA (2-[4-(2-methoxyphenyl) piperazin-1-ylmethyl]imidazo[1,2-a]pyridine) and other selective D4 receptor agonists (Heier et al., 1997; Melis et al., 2006b; Löber et al., 2009), were found able to induce penile erection when injected systemically, i.c.v. and into the paraventricular nucleus, although less effectively than apomorphine. The pro-erectile effect of these D4 receptor agonists was prevented by L-745,870 (3-(4-[chlorophenyl] piperazin-1-yl)-methyl-1H-pyrrolo[2,3-B]pyridine trihydrochloride), a selective D4 receptor antagonist (Patel et al., 1997; Melis et al., 2005, 2006b; Löber et al., 2009).
Finally, the pro-erectile effect of the above D4 receptor agonists was also reduced by nitric oxide-synthase inhibitors, given into the paraventricular nucleus, and by d(CH2)5Tyr(Me)2-Orn8-vasotocin, a selective oxytocin receptor antagonist given i.c.v. but not in the paraventricular nucleus. These results are in line with the hypothesis that D4 receptor agonists also stimulate oxytocinergic neurons by activating nitric oxide-synthase, and releasing oxytocin in extrahypothalamic brain areas, which in turn facilitates penile erection, as shown for apomorphine and classical D2 agonists (Melis et al., 2005, 2006b; Löber et al., 2009).
The above findings also support the hypothesis that dopamine induces penile erection by acting on D4 receptors located on the cell bodies of paraventricular oxytocinergic neurons, and which cause an increased Ca2+ influx into the cell bodies of oxytocinergic neurons, leading to an increased nitric oxide production. Nitric oxide in turn activates oxytocinergic neurons to release oxytocin in extra-hypothalamic brain areas and in the spinal cord, as already discussed. In this regard, it is noteworthy that dopamine receptors have been identified in the cell bodies of oxytocinergic neurons in the paraventricular nucleus only recently by double immuno-fluorescence studies with high selective D2, D3 and D4 receptor antibodies and with oxytocin antibodies. These studies have shown the expression of all three D2receptor subtypes (D2, D3 and D4), which co-localized separately in the cell bodies of oxytocinergic neurons in the paraventricular nucleus (and also in the supraoptic nucleus and the medial preoptic area) (Baskerville and Douglas, 2008; Baskerville et al., 2009).
This provides strong neuroanatomical support to the possibility that dopamine and dopamine receptor agonists of the D2 type induce penile erection by activating directly oxytocinergic neurons projecting to the extrahypothalamic brain areas recalled above, e.g., the spinal cord, the ventral tegmental area, the hippocampus and the amygdala. However, these findings do not provide any help for the identification of the D2 receptor subtype/s, whose stimulation causes the erectile response. Unfortunately, no help can be obtained even from studies aimed at identifying oxytocinergic neurons activated by dopamine receptor agonists in the paraventricular nucleus. Indeed, in spite of the different activity on the various dopamine receptor subtypes, either mixed dopamine receptor agonists (e.g., apomorphine), or selective D2 receptor agonists (e.g., quinpirole, which acts on all D2 receptor subtypes) or selective D4 receptor agonists cause the activation of oxytocinergic neurons, as measured by the increase of FOS protein in parvocellular oxytocinergic neurons of the paraventricular nucleus (Bitner et al., 2006). However, this finding has been recently questioned, as the FOS protein increase in paraventricular oxytocinergic neurons was found only when penile erection was induced by quinerolane, which acts mainly on D2 and D3 receptor subtypes, but not by PD 168077, a D4 receptor agonist, in spite of the ability of both compounds to induce the sexual response (Baskerville et al., 2009).
Further experiments with selective agonists of the other D2 receptor subtypes (mainly D2 and D3) are then necessary to identify the exact role of each dopamine receptor subtype in the control of erectile function at the paraventricular level. In this regard, as already recalled before, apomorphine, which acts potently on all dopamine receptor subtypes (see Brioni et al., 2004, and references therein), is much more effective than D4 receptors agonists in inducing penile erection when injected into the paraventricular nucleus. This might be explained by a higher affinity of apomorphine on D4 receptors when compared to that of the tested D4 receptor agonists, or alternatively, D4 receptor agonists may act as D4 receptor partial agonists, or the concomitant activation of different dopamine receptor subtypes by apomorphine may produce a higher activation of oxytocinergic neurons mediating penile erection, than the activation by D2 receptor agonists of the D4 receptor subtype only.
Interactions between dopamine D1 and D2 receptors have been already described in the control of sexual behaviour at the level of the medial preoptic area (see Hull et al., 1989). In the case that the inability of selective D2 receptor agonists to induce penile erection (Hsieh et al., 2004) will be confirmed (but see Depoortère et al., 2009), for instance even also after injection of these compounds into the paraventricular nucleus, a major role for D3 receptors alone or together with that of D4 receptors in the activation of oxytocinergic neurons mediating penile erection should be analyzed in detail (see Baskerville et al., 2009). Unfortunately, selective D2 and D3 receptor agonists (e.g., which differ in their affinity for these two receptor subtypes for at least four/five orders of magnitude in vitro) are not available at the moment. For this reason, the recent suggestion that D3 receptors mediate penile erection induced by classical D2 receptor agonists, which is based mainly on the ability of putative D3 receptor antagonists characterized in in vitro experiments, to reduce penile erection induced by classical D2 agonists, such as apomorphine, quinpirole and pramipexole, which potently activate all dopamine D2 receptor subtypes (Collins et al., 2009), needs certainly to be validated with other experiments. This validation is necessary also because no effect of D4 receptor agonists on penile erection was found in this study, in striking contrast with the results of the studies cited above, which demonstrate a pro-erectile effect of D4 receptor agonists. Indeed, even the ability of apomorphine to induce penile erection (and yawning) in D4 receptor knockout mice with a potency identical to that seen in wild type D4 receptor knockout mice and the ability of D3 receptor antagonists to abolish the apomorphine response in these animals (Collins et al., 2009) cannot be considered as a definitive evidence for a selective role of the D3 receptor subtype in D2 receptor agonist-induced penile erection. Species differences apart, studies with neurotransmitter/neuropeptide and/or neurotransmitter/neuropeptide receptor gene ablation (neurotransmitter/neuropeptide and/or neurotransmitter/neuropeptide receptor knockout animals) have usually added further confusion and complications in the confirmation of the putative sexual role of neurotransmitters and/or neuropeptides and their receptors. Namely, oxytocin gene ablation produces oxytocin knock out mice that mate and copulate normally, as if oxytocin was unnecessary for mating and copulation. Also the homozygous female oxytocin knockout mice show normal mating and parturition, although with a marked impairment of milk letdown (Nishimori et al., 1996; Young et al., 1996). The ablation of the gene encoding neuronal nitric oxide synthase, also produces nitric oxide synthase knock out mice that mate and copulate normally (Huang et al., 1993). This occurs in spite of the fact that nitric oxide synthase produces nitric oxide, which is one of the main physiological mediators of penile erection at the penile level (Burnett et al., 1992; Rajfer et al., 1992) and at the central level, in the paraventricular nucleus of the hypothalamus (Argiolas, 1994; Benelli et al., 1995; Melis et al., 1998). However, these findings probably indicate an important feature of reproductive physiology, i.e., the redundancy of the systems involved in its control at central and peripheral level.
Such redundancy has certainly an evolutionary origin, since it guarantees the passage of genes to the next generation for the survival of the species. Therefore, the fact that ablation of the D4 receptor gene does not alter the pro-erectile effect of apomorphine, suggests that D4 receptors, like oxytocin and nitric oxide, are only a few of the mediators working in the systems controlling erectile function, rather than suggesting that there is no role for these receptors in the control of penile erection and sexual behaviour. The failure of D4 agonists to induce penile erection when given systemically to male rats of different strains has been recently reported by another study (Depoortère et al., 2009). However, in contrast to the work of Collins et al. (2009), and to make the picture on the role of the different D2 receptor subtypes in the control of penile erection even more puzzling, this study also shows that putative selective D3 receptor antagonists given systemically were unable to reduce apomorphine-induced penile erection in male rats of the strain more sensitive to the pro-erectile effect of apomorphine, while the sexual response (and yawning) was antagonized by the selective D2 antagonist L-741,626 (3-[[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]methyl-1H-indole), leading the authors to suggest that D2 receptors, rather than D3 and D4 receptors, are those playing a major role in D2 agonist-induced penile erection (Depoortère et al., 2009). Finally, the possibility that the excitatory effect of dopamine receptor agonists on oxytocinergic neurons mediating penile erection, at least in part, is indirect rather than direct, e.g., mediated or influenced by changes in the activity of other neurotransmitters neuropeptides able to modulate the activity of oxytocinergic neurons in the paraventricular nucleus, cannot be completely ruled out.
3.2. Glutamic acid-oxytocin interaction in the paraventricular nucleus
The paraventricular nucleus of the hypothalamus is very rich in synapses containing an excitatory amino acid as a neurotransmitter (e.g., glutamic acid and aspartic acid) (Van Den Pol, 1991). Excitatory amino acids in this nucleus are involved in numerous functions, including penile erection and sexual behaviour (Roeling et al., 1991; Melis et al., 1994b, 2000, 2004b). Accordingly, N-methyl-d-aspartic acid (NMDA), a selective agonist of the NMDA receptor subtype, but not (±)-_-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA), a selective agonist of the AMPA receptor subtype or (±)-trans(1)-amino-1,3-cyclopentane dicarboxilic acid (ACPD), a selective agonist of the metabotropic receptor subtype, was found capable of inducing penile erection when injected into the paraventricular nucleus of freely moving rats (Melis et al., 1994b). The pro-erectile effect of 948 M.R. Melis, A. Argiolas / Neuroscience and Biobehavioral Reviews 35 (2011) 939-955 NMDA was prevented by (+)MK-801, a non-competitive NMDA receptor antagonist (Woodruff et al., 1987), injected into the paraventricular nucleus (Melis et al., 1994b). In line with these results, in telemetry studies aimed at monitoring intracavernous pressure, NMDA was found much more active than agonists of the other excitatory amino acid receptor subtypes when injected into the paraventricular nucleus in increasing intracavernosal pressure in awake or anaesthetized male rats (Zahran et al., 2000; Chen and Chang, 2003).
As suggested above for oxytocin and dopamine, it is likely that NMDA receptors mediating penile erection are located in the cell bodies of oxytocinergic neurons, since excitatory amino acid nerve endings impinge on oxytocinergic cell bodies in the paraventricular nucleus (Van Den Pol, 1991). In analogy to what found with dopamine receptor agonists, the pro-erectile effect of NMDA is apparently mediated by the activation of oxytocinergic neurotransmission, being abolished by the oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin given i.c.v., but not into the paraventricular nucleus (see Argiolas and Melis, 1995, 2004, 2005 and references therein). Likewise, NMDA-induced activation of oxytocinergic neurotransmission is also secondary to the activation of nitric oxide-synthase, since NMDA-induced penile erection is prevented by nitric oxide-synthase inhibitors (N-Nitro-N-methyll-arginine methyl ester and N-methyl-thio-l-citrulline) given into the paraventricular nucleus, and NMDA injected into the paraventricular nucleus at doses that induce penile erection, increases nitric oxide production in the hypothalamic nucleus (see Argiolas and Melis, 1995, 2004, 2005 and references therein). As for dopamine receptor agonists, the NMDA induced activation of nitric oxidesynthase may be also secondary to an increased Ca2+ influx in oxytocinergic cell bodies through the Ca2+ channel-coupled NMDA receptors, as shown in several neural preparations (for a review see Snyder, 1992; Southam and Garthwaite, 1993; Schuman and Madison, 1994 and references therein). Nitric oxide in turn activates oxytocinergic neurotransmission (see above). The origin of glutamatergic projections that activate paraventricular oxytocinergic neurons mediating penile erection is unknown, although some neuroanatomical and electrophysiological evidence suggest that they may originate, at least in part, in the hippocampus (Saphier and Feldman, 1987; Chen et al., 1992). Although further work is necessary to characterize better the origin of the glutamatergic projections to the paraventricular nucleus, the involvement of glutamic acid in the paraventricular nucleus in the control of penile erection and sexual behaviour is clearly supported by microdialysis studies. Accordingly, the extra-cellular concentrations of glutamic acid and aspartic acid were increased in the dialysate obtained from the paraventricular nucleus of male rats showing noncontact erections when put in the presence of inaccessible estrogen + progesterone-primed receptive female rats (Melis et al., 2004b), penile erections that are also mediated by the activation of central oxytocinergic transmission (Melis et al., 1999a,b). Such increases were found even higher when copulation with the receptive female was allowed (Melis et al., 2004a). In line with the hypothesis that an increased activity of excitatory amino acids occurs in the paraventricular nucleus during penile erection and copulation, both noncontact erections and copulatory behaviour (during which in copula penile erections occur) are reduced by blockade of NMDA receptors in the paraventricular nucleus, and this reduction is followed by a decrease in the increase of nitric oxide production that occurs in this hypothalamic nucleus in these physiological contexts (Melis et al., 2000). An increase in extracellular glutamic acid concentration secondary to a decreased GABA release from GABAergic nerve endings impinging on excitatory amino acidergic synapses juxtaposed to oxytocinergic cell bodies, was also found in the paraventricular nucleus after the blockade of cannabinoid CB1 receptors by the CB1 antagonist SR 141761A, given into the lateral ventricles or directly into the paraventricular nucleus at doses that induce penile erection (see Succu et al., 2006; Castelli et al., 2007). Such increase led to the activation of nitric oxide-synthase in the cell bodies of oxytocinergic neurons, increasing nitric oxide production. Nitric oxide in turn activates oxytocinergic neurons mediating penile erection as described above. In line with such mechanism, SR 141761A induced penile erection was reduced by the blockade of NMDA receptors and by nitric oxide-synthase inhibitors, but not by the blockade of dopamine or oxytocin receptors in the paraventricular nucleus, while it was prevented by the blockade of central oxytocin receptors by oxytocin receptor antagonists given i.c.v. (Melis et al., 2004a, 2006a).
3.3. Oxytocin-dopamine interaction in the ventral tegmental area
Oxytocin induces penile erection when injected into the caudal part of the ventral tegmental area in a dose-dependent manner (Melis et al., 2007). The lowest active dose injected unilaterally was 20 ng, while the highest dose tested was 100 ng. The oxytocin effect is mediated by the activation of oxytocinergic receptors, as the sexual response is abolished by the prior injection of the oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin in the caudal ventral tegmental area. These receptors are localized in the cell bodies of dopaminergic neurons, which project mainly to the shell of the nucleus accumbens. Accordingly, (1) double immuno-fluorescence studies show that in the caudal ventral tegmental area oxytocinergic fibres are in close contact with the cell bodies of dopaminergic neurons, the majority of which were positively labeled for tyrosine-hydroxylase and containing the retrograde tracer Fluorogold previously injected into the shell of the nucleus accumbens (Melis et al., 2007), and (2) ventral tegmental area oxytocin-induced penile erection occurs concomitantly with an increase in the concentration of extra-cellular dopamine in the dialysate obtained from the shell of the nucleus accumbens (Melis et al., 2007). Oxytocin-induced penile erection also occurs concomitantly with an increase in nitric oxide production in the ventral tegmental area, being both responses antagonized not only by d(CH2)5Tyr(Me)2-Orn8-vasotocin and by the nitric oxide synthase inhibitor S-methyl-thio-l-citrulline, but also by _-conotoxin, a voltage-dependent Ca2+ channels blocker, and by ODQ (1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one), a potent inhibitor of guanylate cyclase, all given in the caudal ventral tegmental area before oxytocin (Succu et al., 2008). As many of the Fluorogold labeled dopaminergic cell bodies contacted by oxytocinergic fibres, found to be positive for tyrosine hydroxylase in the caudal ventral tegmental area, were also positively labeled for nitric oxide-synthase and guanylate cyclase (Succu et al., 2008), oxytocin-induced penile erection may be mediated by the following mechanism. The activation of oxytocinergic receptors in dopaminergic cell bodies by the peptide increases Ca2+ influx inside the cell bodies of dopaminergic neurons. This activates nitric oxide-synthase, a Ca2+-calmodulin-dependent enzyme, thereby increasing nitric oxide production. Nitric oxide in turn activates guanylate cyclase, leading to an increased concentration of cyclic GMP. Cyclic GMP activates dopaminergic neurons projecting to the nucleus accumbens. The role of cyclic GMP in penile erection induced by oxytocin injected into the caudal ventral tegmental area is also supported by the ability of 8-bromo-cyclic GMP, an active phosphodiesterase-resistant analogue of cyclic GMP, to induce penile erection when injected into the caudal ventral tegmental area, and to increase extra-cellular dopamine in the dialysate from the nucleus accumbens (Succu et al., 2008; Melis et al., 2009a). This is at variance with the mechanism by which nitric oxide activates oxytocinergic neurons in the paraventricular nucleus, being 8-bromo-cyclic GMP unable to induce penile erection when injected in this nucleus (Melis and Argiolas, 1995b) (Fig. 2). The role of dopamine released in the nucleus accumbens in penile erection induced by oxytocin injected into the caudal ventral tegmental area is supported by the ability of haloperidol, a potent dopamine receptor antagonist injected into the nucleus accumbens to reduce the oxytocin response (Melis et al., 2007). As to the neural pathways activated by dopamine in the nucleus accumbens leading to penile erection, these are still unknown. However, the available data suggest that these pathways activate dopamine neurotransmission in the paraventricular nucleus of the hypothalamus. Accordingly, oxytocin-induced penile erection occurs concomitantly to an increase in extra-cellular dopamine not only in the nucleus accumbens, but also in the paraventricular nucleus, and is antagonized by the dopamine receptor antagonist haloperidol injected into the paraventricular nucleus (Melis et al., 2007). All together, these results support the idea that oxytocinergic neurons originating in the paraventricular nucleus and projecting to the caudal ventral tegmental area, when activated release oxytocin in this area, thereby activating a NO-cyclic GMP signaling system, which in turn activates mesolimbic dopaminergic neurons (Melis et al., 2007, 2009a; Succu et al., 2008). Dopamine released in the nucleus accumbens in turn activates neural pathways leading to the activation of incerto-hypothalamic dopaminergic neurons, which stimulate paraventricular oxytocinergic neurons projecting to the spinal cord mediating penile erection. At the same time, dopamine released in the paraventricular nucleus might also activate oxytocinergic neurons projecting to extra-hypothalamic brain areas such as the ventral tegmental area, the hippocampus, the amygdala and perhaps other brain areas.
As recalled above, in line with this hypothesis, apomorphine injected into the paraventricular nucleus at a dose that induces penile erection also increases extra-cellular dopamine concentration in the nucleus accumbens, an effect reduced by the oxytocin receptor antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin injected into the ventral tegmental area (Succu et al., 2007; Melis et al., 2009a). Together, the above neural pathways might constitute a hypothetical neural circuitry involving dopamine, oxytocin and other neurotransmitters (e.g., glutamic acid, see below) influencing not only sexual performance, but also sexual motivation and sexual rewarding (see Section 4).
3.4. Oxytocin-glutamic acid interaction in the ventral subiculum of the hippocampus
Oxytocin induces penile erection when injected into the ventral, but not in the dorsal subiculum, in a dose dependent manner (Melis et al., 2009b). The sexual response is mediated by the stimulation of oxytocin receptors, being abolished by the prior injection of d(CH2)5Tyr(Me)2-Orn8-vasotocin given into the same site of oxytocin, as found in other brain areas (see above). As to the localization of these receptors, the available data suggest that they are localized in the cell bodies of neurons rich in nitric oxide-synthase.
Accordingly, microdialysis studies show that oxytocin-induced penile erection occurs concomitantly with an increase in nitric oxide production in the ventral subiculum, and this increase is abolished not only by the prior injection of the nitric oxidesynthase inhibitor S-methyl-thio-l-citrulline and by the nitric oxide scavenger haemoglobin, but also by d(CH2)5Tyr(Me)2-Orn8-vasotocin, all given into the same site of oxytocin at doses that antagonize penile erection (Melis et al., 2010). More important, oxytocin-induced penile erection occurs also concomitantly with an increase in the concentration of extra-cellular glutamic acid in the ventral subiculum, which is only partially antagonized by the non-competitive NMDA receptor antagonist (+)MK-801 given into the ventral subiculum (Melis et al., 2010). Together, these results suggest that newly formed nitric oxide, by acting as an intercellular messenger, activates glutamic acid neurotransmission leading to penile erection, possibly through neural efferent projections from the ventral subiculum to extra-hippocampal brain areas. In line with this hypothesis, NMDA injected into the ventral subiculum induces penile erection in a dose-dependent manner, and this effect is antagonized completely by the prior injection into the same site of (+)MK-801, but not by S-methyl-thio-l-citrulline, haemoglobin or d(CH2)5Tyr(Me)2-Orn8-vasotocin (Melis et al., 2010). As to the neural efferent pathways projecting to extra-hippocampal brain areas activated by excitatory amino acids (i.e., glutamic acid) in the ventral subiculum, it is likely that these are glutamatergic, as are the majority of hippocampal efferent projections. At the moment, it might be only suggested that these projections modulate the activity of mesolimbic dopaminergic neurons, which in turn modulate the activity of incerto-hypothalamic dopaminergic neurons in the paraventricular nucleus, leading to the activation of oxytocinergic neurons mediating penile erection as already discussed (see above).
Accordingly penile erection induced by ventral subiculum oxytocin occurs concomitantly with an increase in the concentration of extra-cellular dopamine in the shell of the nucleus accumbens, and this increase, like penile erection, is abolished by d(CH2)5Tyr(Me)2-Orn8-vasotocin given into the ventral subiculum before oxytocin (Melis et al., 2009b). Moreover, since ventral subiculum oxytocin induced penile erection is also reduced by (+)MK-801 injected into the ventral tegmental area, but not into the nucleus accumbens (Melis et al., 2009b) and occurs concomitantly to an increase of extra-cellular glutamic acid in the ventral tegmental area, but not in the nucleus accumbens, being both responses abolished by d(CH2)5Tyr(Me)-Orn8-vasotocin, injected into the ventral subiculum before oxytocin (see Fig. 3), it is likely that the activation of mesolimbic dopaminergic neurons is secondary to an increased glutamatergic neurotransmission in the ventral tegmental area. This suggests that a glutamic acid-dopamine interaction controlling penile erection exists in the ventral tegmental area. Further studies are necessary to ascertain whether the pro-erectile efferent glutametergic pathways from the subiculum to the ventral tegmental area are direct or indirect, i.e., through the prefrontal cortex or other brain areas (see Melis et al., 2009b and references therein). Since the paraventricular nucleus also receives glutamatergic projections from the hippocampus (see above and Saphier and Feldman, 1987), and glutamic acid activates paraventricular oxytocinergic neurons including those projecting to the ventral tegmental area (see Argiolas and Melis, 2005 and references therein), and oxytocin in the ventral tegmental area induces penile erection and increases the activity of mesolimbic dopaminergic neurons (see above), it is tempting to speculate that paraventricular oxytocinergic neurons may be also involved, at least in part, in the activation of mesolimbic dopaminergic neurons by oxytocin injected into the ventral subiculum (see Section 4).
4. Concluding remarks
The studies reviewed above confirm and extend early findings showing that in male rats oxytocin plays a key role in the central control of penile erection at the level of the paraventricular nucleus of the hypothalamus and of the spinal cord. In particular, the most recent studies show that oxytocin influences penile erection also by acting in other brain areas, i.e., the ventral tegmental area, the ventral subiculum, and the posteromedial cortical nucleus of the amygdala.
At the paraventricular level, the most important new finding is perhaps the discovery of the expression of all dopamine receptors of the D2 family (D2, D3 and D4) in the cell bodies of oxytocinergic neurons in the paraventricular nucleus (and in the supraoptic nucleus and the medial preoptic area) (Baskerville and Douglas, 2008; Baskerville et al., 2009). This provides strong neuroanatomical support to the hypothesis that dopamine and dopamine receptor agonists may activate directly oxytocinergic neurons involved in erectile function and projecting not only to the spinal cord, but also to extra-hypothalamic brain areas. In this regard, it is also important the discovery that dopamine receptor agonist-induced increase in intracavernous pressure is reduced by the blockade of oxytocinergic receptors in the lumbo-sacral spinal cord (Baskerville et al., 2009). Indeed, although such evidence has been obtained in anaesthetized male rats, the finding confirms the activation of a paraventriculo-spinal oxytocinergic descending pathway involved in dopamine receptor agonist-induced penile erection. However, it has still to be ascertained whether penile erection induced by the stimulation of dopamine receptors present in oxytocinergic cell bodies is secondary to the activation of a specific dopamine receptor subtype of the D2 family (D2, D3 or D4) or if these receptor subtypes co-operate in modulating the erectile response, possibly in different ways depending on the context in which penile erection occurs (see Moreland et al., 2004; Enguehard-Gueiffier et al., 2006; Melis et al., 2006a,b; Löber et al., 2009; Collins et al., 2009; Depoortère et al., 2009; Baskerville et al., 2009).
Another important new finding is that oxytocin induces penile erection when injected not only into the paraventricular nucleus or the CA1 field of the hippocampus, but also into the ventral tegmental area, the ventral subiculum, and the posteromedial cortical nucleus of the amygdala. These brain areas were not tested in the earlier studies showing that oxytocin increased spontaneous penile erection episodes in male rats, although they receive like the lumbo-sacral spinal cord oxytocinergic projections from the paraventricular nucleus. Oxytocin was indeed found capable of increasing spontaneous penile erection episodes, which occur in adult male rats in the absence of any sexual stimuli, such as those which originate from the presence of an accessible or inaccessible receptive (estrogen-progesterone primed) ovariectomized female rat or manipulation of the genitalia,wheninjected into the paraventricular nucleus and the CA1 field of the hippocampus, but not in the dorsal subiculum, the lateral septum, the caudate nucleus, the medial preoptic area, the ventromedial nucleus and the supraoptic nucleus (Melis et al., 1986). In all these studies penile erection was usually counted when the penis emerged from the penile sheath by an observer who was unaware of the given treatments directly during the experiment or later by observing the experiment recorded on a videotape with a video camera apparatus. Each penile erection episode lasts for 0.5-1 min and is usually accompanied by penile grooming and/or hip flexions. No experiment is usually done in these rats to ascertain the effect of sexual experience, age or if these rats can be divided in low or high responders to the pro-erectile effect of oxytocin injected into the different brain areas. This applies also to the majority of studies on the pro-erectile effect of other neuropeptides and drugs that increase spontaneous penile erection episodes, including dopamine agonists, excitatory amino acids, ACTH-MSH, hexarelin and VGF peptides. However, the pro-erectile effect of these compounds has been repeatedly confirmed by telemetry methods, which determine the occurrence of penile erection by the increase in intracavernous pressure that occurs spontaneously or after administration of these compounds by different routes, i.e., systemically, intracerebroventricularly or directly into specific brain nuclei, after the implant of a pressure microtransducer directly into the cavernous corpora (see Bernabè et al., 1999). In the ventral tegmental area, the ventral subiculum and the posteromedial nucleus of the amygdala also oxytocin induces penile erection by acting on oxytocinergic receptors. This leads to the activation of mesolimbic dopaminergic neurons originating in the ventral tegmental area and projecting to the shell of the nucleus accumbens, as measured by the increases in extra-cellular dopamine concentration in the dialysate obtained from the shell of nucleus accumbens and by the reduction in the erectile response induced by the peptide injected into these extra-hypothalamic areas, found after the blockade of dopaminergic receptors in the nucleus accumbens (see below). As to the mechanisms activated by the stimulation of oxytocinergic receptors in these brain areas, which lead to the activation of mesolimbic dopaminergic neurons and to penile erection, the best clarified are those occurring in the caudal ventral tegmental area. Indeed, here pharmacological and immuno-fluorescence results show that oxytocin nerve endings impinge on the cell bodies of dopaminergic neurons projecting to the shell of the nucleus accumbens (Melis et al., 2007, 2009a; Succu et al., 2008). Many of these neurons are rich in nitric oxide synthase and in guanylate cyclase. The stimulation of oxytocinergic receptors in the cell bodies of these dopaminergic neurons causes the activation of nitric oxide synthase leading to an increased nitric oxide production. Nitric oxide in turn activates guanylate cyclase, thereby increasing the concentration of cyclic GMP, which leads to the activation of mesolimbic dopaminergic neurons and to the release of dopamine in the nucleus accumbens, as measured by the increase in extra-cellular dopamine in the dialysate from the nucleus accumbens obtained by intra-cerebral microdialysis (Succu et al., 2008). Dopamine released in the nucleus accumbens in turn activates neural pathways leading to penile erection. This is supported by the ability of dopamine receptor antagonists haloperidol and/or cis-flupentixol injected into the nucleus accumbens to reduce ventral tegmental area oxytocin-induced penile erection (Succu et al., 2008). One of the pro-erectile pathways seems to activate incerto-hypothalamic dopaminergic neurons, in particular those that project to the cell bodies of paraventricular oxytocinergic neurons. Indeed oxytocin injected into the caudal ventral tegmental area increases extracellular dopamine not only in the nucleus accumbens but also in the paraventricular nucleus, and the blockade of dopamine receptors in the paraventricular nucleus reduces significantly ventral tegmental area oxytocin-induced penile erection (Succu et al., 2007, 2008; Melis et al., 2007, 2009a). The existence of these nucleus accumbens dopamine-paraventricular dopamine-paraventricular oxytocin-ventral tegmental area oxytocin-dopamine links is also suggested by the ability of a pro-erectile dose of apomorphine and of the D4 receptor agonist PD 168077 injected into the paraventricular nucleus to increase extra-cellular dopamine in the shell of the nucleus accumbens (Succu et al., 2007), response that is abolished by d(CH2)5Tyr(Me)-Orn8-vasotocin given into the ventral tegmental area (Melis et al., 2009a, see also below). However, further work is necessary to identify the neural pathways that connect the nucleus accumbens to the incerto-hypothalamic dopaminergic system.
The mechanism by which oxytocin induces penile erection and activates mesolimbic dopaminergic neurons when injected into the ventral subiculum or into the posteromedial nucleus of the amygdala is only partially understood at the moment. In these areas also, oxytocin activates its own receptors which lead to the activation of nitric oxide-synthase, thereby increasing nitric oxide production. Nitric oxide in turn activates unknown efferent projections, which apparently increase glutamatergic neurotransmission in the ventral tegmental area. Glutamic acid then stimulates mesolimbic dopaminergic neurons leading to penile erection. This hypothesis is supported mainly by the ability of oxytocin injected into the ventral subiculum to increase extra-cellular glutamic acid in the ventral tegmental area (Fig. 3), and of the non-competitive NMDA receptor antagonist (+)MK-801 injected into the ventral tegmental area, but not in the nucleus accumbens, to reduce penile erection induced by oxytocin injected either into the ventral subiculum or into the posteromedial nucleus of the amygdala (Melis et al., 2009b). At the moment, more details are available for the ventral subiculum oxytocin-induced penile erection. Here oxytocin-induced penile erection seems secondary to the activation of oxytocinergic receptors located in the cell bodies of nitric oxide-synthase-containing neurons. This causes an increase in the production of nitric oxide, which activates glutamatergic neurotransmission by acting as an intercellular messenger with a mechanism similar to that described for long term potentiation (see Snyder, 1992; Southam and Garthwaite, 1993; Schuman and Madison, 2004). In line with this hypothesis oxytocin-induced penile erection occurs concomitantly with an increase in extracellular glutamic acid in the dialysate from the ventral subiculum, and stimulation of excitatory amino acid receptors in the ventral subiculum by NMDA, induces penile erection. Glutamic acid in turn activates neural efferent projections, which lead to the activation of mesolimbic dopaminergic neurons in the ventral tegmental area, as above reported. If these mechanisms are operative also in the posteromedial nucleus of the amygdala is unknown at present. Moreover, further studies are necessary to prove that (1) oxytocinergic nerve endings and receptors in the ventral subiculum and in the posteromedial nucleus of the amygdala are localized in cell bodies of neurons containing nitric oxide-synthase, (2) if these neurons are eventually under the control of excitatory amino acid (glutamatergic) synapses and (3) to identify the signaling system activated by nitric oxide. In this regard, it is noteworthy that the ability of oxytocin to increase nitric oxide-synthase activity in the cell bodies of dopaminergic neurons in the caudal ventral tegmental area and of still unknown neurons in the ventral subiculum and the posteromedial nucleus of the amygdala, recalls the ability of oxytocin to activate nitric oxide-synthase in the cell bodies of oxytocinergic neurons in the paraventricular nucleus (Melis et al., 1997). However, while in the ventral tegmental area the increased production of nitric oxide in the cell bodies of dopaminergic neurons leads to the activation of these neurons by activating guanylate cyclase and increasing cyclic GMP, this does not occur in the paraventricular nucleus. Accordingly, 8-bromo-cyclic GMP injected into the paraventricular nucleus does not induce penile erection, while it does so when injected into the ventral tegmental area. Another signaling pathway different from the nitric oxidecyclic GMP system is then involved at the paraventricular level in the activation of oxytocinergic neurons mediating penile erection by endogenous and/or exogenous nitric oxide (Melis and Argiolas, 1995b; Melis et al., 1997) (Fig. 1). On the other hand, cyclic GMP in the ventral tegmental area seems to play also a key role in the activation of mesolimbic dopaminergic neurons and in the increase in extra-cellular dopamine occurring in the dialysate obtained from the shell of the nucleus accumbens of male rats selected for showing or not showing noncontact penile erections when put in the presence of an inaccessible ovariectomized receptive (oestrogen + progesterone treated) female rat. In these experimental conditions, in male rats showing noncontact penile erections, an increase in extra-cellular dopamine concentration is found as expected, and this increase is further increased, although only modestly, by phosphodiesterase inhibitors given into the caudal ventral tegmental area (Sanna et al., 2009). In contrast, in male rats not showing noncontact erections, in which no appreciable increase in extra-cellular dopamine in the nucleus accumbens is found, extracellular dopamine concentration is found markedly increased by phosphodiesterase inhibitors given into the caudal ventral tegmental area and noncontact erections are observed (Sanna et al., 2009).
The ability of oxytocin injected into the ventral tegmental area, in the ventral subiculum and in the posteromedial cortical nucleus of the amygdala, together with that of dopamine agonists injected into the paraventricular nucleus, to induce penile erection and to activate mesolimbic dopaminergic neurons deserves some comment. First, mechanisms similar to those recalled above may be operative when penile erection occurs in physiological contexts, such as during copulation (when in copula penile erections occur) or during noncontact penile erections. These erections are pheromone-mediated penile erections indistinguishable from those induced by drugs or oxytocin, which occur when sexually potent male rats are put into the presence of an inaccessible receptive (ovariectomized estrogen + progesterone primed) female rat and are considered as an index of sexual arousal (Sachs, 1997, 2007). Indeed, although these results do not demonstrate that oxytocin in these areas plays a role in penile erection occurring in physiological contexts or after drug administration, they add further strength to early findings suggesting that these brain areas belong to those where oxytocin given centrally acts to increase not only spontaneous penile erection episodes seen after pro-erectile drugs, but also to improve male (and female) sexual behaviour (see Argiolas and Melis, 2004 and references therein). Accordingly, oxytocin concentration increases in the hippocampus of male rats treated with a pro-erectile dose of apomorphine, a classical dopamine agonist (Melis et al., 1990) and d(CH2)5Tyr(Me)-Orn8-vasotocin, which blocks oxytocin receptors, is extremely effective not only in impairing copulatory behaviour (Argiolas et al., 1987a) but also the facilitatory effect of apomorphine on male copulatory behaviour (Argiolas et al., 1987b) in sexually potent male rats, during which in copula penile erection occur. d(CH2)5Tyr(Me)-Orn8-vasotocin is also extremely potent in reducing noncontact erections in sexually potent male rats, when given in nanogram amounts into the lateral ventricles, but not into the PVN (Melis et al., 1999a).
Second, mesolimbic dopaminergic neurons play a key role in the motivational and rewarding properties of natural reinforcing stimuli, such as food, water and sexual activity (Fibiger and Phillips, 1988; Wise and Rompre, 1989; Everitt, 1990). In particular, dopamine released from these neurons is thought to mediate the transposition of the motivational aspects of natural stimuli into goal directed behaviours, for instance in the case of sexual activity, the seeking of a sexual partner and of sexual intercourse to reach reward and satisfaction (Goto and Grace, 2005). Accordingly, extracellular dopamine concentration increases in dialysate from the nucleus accumbens of sexually potent male rats during exposure to an inaccessible ovariectomized estrogen + progesterone-primed receptive female rat, and such increase was even higher when the male rat was allowed to copulate with the receptive female (Pfaus and Everitt, 1995).
Third, the present results support the hypothesis that a neural circuit connects the paraventricular nucleus with the ventral tegmental area directly or indirectly (through the ventral subiculum and/or the posteromedial cortical nucleus of the amygdala) and the nucleus accumbens, and from here through unknown pathways back again to the paraventricular nucleus to control the activity of oxytocinergic neurons projecting to the spinal cord mediating penile erection and of oxytocinergic neurons projecting to the ventral tegmental area, the ventral subiculum and the posteromedial cortical nucleus of the amygdala, modulating in this way the activity of mesolimbic dopaminergic neurons (Fig. 4). This complex neural circuit may play a role in the integration of neural activities involved in the control of the consummatory (erectile-ejaculatory) and anticipatory (motivational and rewarding) aspects of male sexual behaviour in physiological contexts. Indeed, extra-cellular dopamine increases in the nucleus accumbens (Pfaus and Everitt, 1995) and in the paraventricular nucleus of sexually potent male rats during exposure to an inaccessible receptive female rat, when noncontact erections occur, and even more when copulation is allowed, e.g., when in copula penile erections occur (Melis et al., 2003). Thus, although further studies are necessary to clarify the role of endogenous oxytocin in the ventral tegmental area, the ventral subiculum and the amygdala during noncontact erections and sexual behaviour, it may be then reasonable to assume that this hypothetical neural circuit, while contributing to the consummatory aspects of sexual behaviour, in the same time may also activate the mesolimbic dopaminergic system providing a neural substrate for explaining the rewarding properties of sexual activity (Everitt, 1990; Pfaus and Everitt, 1995). In this regard, it is noteworthy that the mesolimbic dopaminergic system activated by oxytocin injected into the ventral tegmental area is the same activated by drugs of abuse such as opiates, cannabinoids, amphetamine, cocaine and alcohol (Tanda et al., 1997), and that oxytocin was found capable of reducing tolerance and dependence to cocaine, morphine, alcohol and cannabinoids (Kovacs et al., 1998; Cui et al., 2001). In conclusion, it seems that oxytocin released not only in the ventral tegmental area, but also in the ventral subiculum and the posteromedial cortical nucleus of the amygdala, can activate mesolimbic dopaminergic neurons, which may be involved in the appetitive and rewarding effects of sexual activity. The activation of mesolimbic dopaminergic neurons may be direct, through oxytocinergic receptors in the cell bodies of mesolimbic dopaminergic neurons, or indirect through the activation of glutamic acid neurotransmission in the ventral tegmental area.
Dopamine released in the nucleus accumbens shell modulates in turn the activity of the incerto-hypothalamic dopaminergic neurons in the paraventricular nucleus causing either penile erection (via activation of oxytocinergic neurons projecting to the spinal cord), or sexual motivation and reward (via activation of oxytocinergic neurons projecting to the ventral tegmental area, the ventral subiculum or the posteromedial cortical nucleus of the amygdala). Since dopamine is also released in the nucleus accumbens shell and in the paraventricular nucleus when penile erection occurs in physiological contexts (e.g., noncontact erections and copulation) (Succu et al., 2007; Melis et al., 2003, 2007), it is likely that central oxytocinergic neurons participate in neural circuits mediating an interaction between the mesolimbic and the incerto-hypothalamic dopaminergic system. These neural circuits may play a role not only in the consummatory phase of sexual behaviour (e.g., penile erection and copulation), but also in sexual motivation, sexual arousal and sexual reward.
Schematic representation of a hypothetical neural circuitry involving oxytocin influencing sexual motivation, rewarding and sexual performance, as suggested by the results of this chapter and previous reports. Oxytocinergic neurons originating in the paraventricular nucleus and projecting to the spinal cord when activated for instance by dopamine and glutamic acid (but also by other neurotransmitters and/or neuropeptides), facilitate penile erection and sexual performance by activating oxytocinergic neurons projecting to the spinal cord. Dopamine and glutamic acid (but also neurotransmitters and neuropeptides) in the paraventricular nucleus also activates oxytocinergic neurons projecting to the ventral tegmental area, thereby activating mesolimbic dopaminergic neurons projecting to the nucleus accumbens, modulating sexual motivation and reward. Dopamine released in the nucleus accumbens (NAs) activates in turn still unknown neural pathways, which increase the activity of incerto-hypothalamic dopaminergic neurons (originating in the A13-A14 groups of Dahlstrom and Fuxe) impingingamongothers on oxytocinergic neurons, including those that project to the spinal cord, leading to penile erection. This circuitry may be also activated by oxytocin injected not only into the caudal ventral tegmental area, but also in the ventral subiculum and in the amygdala, which also receive an oxytocinergic innervation from the paraventricular nucleus, possibly through direct or indirect glutamatergic efferent to the ventral tegmental area, leading to the modulation of both sexual motivation and penile erection. Finally, the above circuitry may be also activated by sexual stimuli and pheromones, since extra-cellular dopamine and glutamic acid increase in the paraventricular nucleus (and in the medial preoptic area) during pheromone-mediated noncontact erections and copulation (for appropriate references see the References list).
Acknowledgements This work was partially supported by a grant from the Italian Ministry of the University and of Research to AA and MRM
References
Andersson, K.E., 2001. Pharmacology of penile erection. Pharmacol. Rev. 53, 417-450. Argiolas, A., 1994. Nitric oxide is a central mediator of penile erection. Neuropharmacology 33, 1339-1344. Argiolas, A., 1999. Neuropeptides and sexual behaviour. Neurosci. Biobehav. Rev. 23, 1127-1142. Argiolas, A., Gessa, G.L., 1991. Central functions of oxytocin. Neurosci. Biobehav. Rev. 15, 217-231. Argiolas, A., Melis, M.R., 1995. Neuromodulation of penile erection: an overview of the role of neurotransmitters and neuropeptides. Prog. Neurobiol. 47, 235-255. Argiolas, A., Melis, M.R., 2004. The role of oxytocin and the paraventricular nucleus in the sexual behaviour of male mammals. Physiol. Behav. 83, 309-317. Argiolas, A., Melis, M.R., 2005. Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog. Neurobiol. 76, 1-21. Argiolas, A., Collu, M., Gessa, G.L., Melis, M.R., Serra, G., 1988. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin antagonizes male copulatory behaviour in rats. Eur. J. Pharmacol. 149, 389-392. Argiolas, A., Collu, M., D’Aquila, P., Gessa, G.L., Melis, M.R., Serra, G., 1989. Apomorphine stimulation of male copulatory behavior is prevented by the oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin in rats. Pharmacol. Biochem. Behav. 33, 81-83. Argiolas, A., Melis, M.R., Gessa, G.L., 1985. Intraventricular oxytocin induces yawning and penile erection in rats. Eur. J. Pharmacol. 117, 395-396. Argiolas, A., Melis, M.R., Gessa, G.L., 1986. Oxytocin: an extremely potent inducer of penile erection and yawning in male rats. Eur. J. Pharmacol. 130, 265-272. Argiolas, A., Melis, M.R., Mauri, A., Gessa, G.L., 1987a. Paraventricular nucleus lesion prevents yawning and penile erection induced by apomorphine and oxytocin but not by ACTH in rats. Brain Res. 421, 349-352. Argiolas, A., Melis, M.R., Vargiu, L., Mauri, A., Gessa, G.L., 1987b. d(CH2)5Tyr(Me)-Orn8-vasotocin, a potent oxytocin antagonist, antagonizes penile erection and yawning induced by oxytocin and apomorphine, but not by ACTH 1-24. Eur. J. Pharmacol. 134, 221-224. Argiolas, A., Melis, M.R., Stancampiano, R., Gessa, G.L., 1990. _-Conotoxin prevents apomorphine and oxytocin-induced penile erection and yawning in male rats. Pharmacol. Biochem. Behav. 37, 253-257. Arletti, R., Bertolini, A., 1985. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides 6, 247-255. Arletti, R., Bazzani, C., Castelli, M., Bertolini, A., 1985. Oxytocin improves male copulatory performance in rats. Horm. Behav. 19, 14-20. Arletti, R., Benelli, A., Bertolini, A., 1990. Sexual behavior of aging male rats is stimulated by oxytocin. Eur. J. Pharmacol. 179, 377-382. Arletti, R., Calzà, L., Giardino, L., Benelli, A., Cavazzutti, E., Bertolini, A., 1997. Sexual impotence is associated with a reduced production of oxytocin and an increased production of opioid peptides in the paraventricular nucleus of the hypothalamus. Neurosci. Lett. 233, 65-68. Bancila, M., Giuliano, F., Rampin, O., Mailly, P., Brisorgueil, M.J., Clas, A., Verge, D., 2002. Evidence for a direct projection from the paraventricular nucleus of the hypothalamus to putative serotoninergic neurons of the nucleus paragigantocellularis involved in the control of erection in rats. Eur. J. Neurosci. 16, 1240-1249. Burnett, A.L., Lowenstein, C.J., Bredt, D.S., Chang, T.S.K., Snyder, S.H., 1992. Nitric oxide: a physiological mediator of penile erection. Science 257, 401-403. Baskerville, T.A., Douglas, A.J., 2008. Interaction between dopamine and oxytocin in the control of sexual behaviour. Prog. Brain Res. 170, 277-289. Baskerville, T.A., Allard, J., Wayman, C., Douglas, A.J., 2009. Dopamine-oxytocin interaction in penile erection. Eur. J. Neurosci. 30, 2151-2164. Benelli, A., Bertolini, A., Poggioli, R., Cavazzutti, E., Calzà, L., Giardino, L., Arletti, R., 1995. Nitric oxide is involved in male sexual behaviour of rats. Eur. J. Pharmacol. 294, 505-510. Bernabè, J., Rampin, O., Sachs, B.D., Giuliano, F., 1999. Intracavernous pressure during erection in rats: an integrative approach based on telemetric recording. Am. J. Physiol. 276, R441-R449. Bitner, R.S., Nikkel, A.L., Otte, S., Martino, B., Barlow, E.H., Bhatia, P., Stewart, A.O., Brioni, J.D., Decker, M.W.,Moreland, R.B., 2006. Dopamine D4 receptor signalling in the rat paraventricular hypothalamic nucleus: evidence of natural coupling involving immediate early gene induction and mitogen activated protein kinase phosphorylation. Neuropharmacology 50, 521-531. Brioni, J.D., Moreland, R.B., Cowart, M., Hsieh, G.C., Stewart, A.O., Hedlund, P., Donnelly-Roberts, D.L., Nakane, M., Lynch 3rd., J., Kolasa, T., Polakowski, J.S., Osinski, M.A., Marsh, K., Andersson, K.E., Sullivan, J.P., 2004. Activation of dopamine D4 receptors by ABT-724 induces penile erection in rats. Proc. Natl. Acad. Sci. U.S.A. 101, 6758-6763. Buijs, R.M., 1978. Intra- and extra-hypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res. 192, 423-435. Buijs, R.M., Geffard, M., Pool, C.W., Hoorneman, E.M.D., 1984. The dopaminergic innervation of the supraoptic and paraventricular nucleus. A light and electron microscopical study. Brain Res. 323, 65-72. Caldwell, J.D., Prange, A.J., Pedersen, C.A., 1986. Oxytocin facilitates the sexual receptivity of oestrogen-treated female rats. Neuropeptides 7, 175-189. Cameron, J.L., Pomerantz, S.M., Layden, L.M., Amico, J.A., 1992. Dopaminergic stimulation of oxytocin concentrations in the plasma of male and female monkeys by apomorphine and a D2 receptor agonist. J. Clin. Endocrinol. Metab. 75, 855-860. Canteras, N.S., Simerly, R.B., Swanson, L.W., 1995. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J. Comp. Neurol. 360, 213-245. Carmichael, M.S., Humbert, R., Dixen, J., Palmisano, G., Greeleaf, W., Davidson, J.M., 1987. Plasma oxytocin increases in human sexual response. J. Clin. Endocrinol. Metab. 64, 27-31. Carter, C.S., 1992. Oxytocin and sexual behaviour. Neurosci. Biobehav. Rev. 16, 131-144. Carter, C.S., Lederhendler, I.I., Kirkpatrick, B., 1997. The Interactive Neurobiology of Affiliation, Annals of the New York Academy of Sciences, vol. 807. The New York Academy of Sciences, New York. Castelli, M.P., Piras, A.P., Melis, T., Succu, S., Sanna, F., Melis, M.R., Collu, S., Ennas, M.G., Diaz, G., Mackie, K., Argiolas, A., 2007. Cannabinoid CB1 receptors in the paraventricular nucleus and central control of penile erection: immunocytochemistry, autoradiography and behavioural studies. Neuroscience 147, 197-206. Chen, K.K., Chang, L.S., 2003. Effect of excitatory amino acid receptor agonists on penile erection after administration into paraventricular nucleus of the hypothalamus in rats. J. Urol. 62, 575-580. Chen, K.K., Chan, J.Y.H., Chang, L.S., Chen, M.T., Chang, S.H.H., 1992. Elicitation of penile erection following activation of the hippocampal formation in the rat. Neurosci. Lett. 141, 218-222. Chen, K.K., Chan, J.Y.H., Chang, L.S., 1999. Dopaminergic neurotransmission at the paraventricular nucleus of the hypothalamus in central regulation of penile erection in the rat. J. Urol. 162, 237-242. Collins, G.T., Truccone, A., Haji-Abdi, F., Newman, A.H., Grundt, P., Rice, K.C., Husbands, S.M., Greedy, B.M., Enguehard-Gueiffer, C., Gueiffer, A., Chen, J., Wang, S., Katz, J.L., Grandy, D.K., Sunahara, R.K., Woods, J.H., 2009. Pro-erectile effects of dopamineD2-like agonists are mediated by the D3 receptor in rats and mice. J. Pharmacol. Exp. Ther. 329, 210-217. Coolen, L.M., Allard, J., Truitt, W.A., McKenna, K.E., 2004. Central regulation of ejaculation. Physiol. Behav. 83, 203-215. Cui, S.S., Bowen, R.C., Gu, G.B., Hannesson, B.K., Yu, P.H., Zhang, X., 2001. Prevention of cannabinoid withdrawal syndrome by lithium: involvement of oxytocinergic neuronal activation. J. Neurosci. 21, 9867-9876. Dahlstrom, A., Fuxe, K., 1964. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brainstem neurons. Acta Physiol. Scand. 62 (Suppl. 232), 1-54. Depoortère, R., Bardin, L., Rodrigues, M., Abrial, E., Aliaga, M., Newman-Tancredi, A., 2009. Penile erection and yawning induced by dopamine D2-like receptor agonists in rats: influence of strain and contribution of dopamine D2, but not D3 and D4 receptors. Behav. Pharmacol. 20, 303-311. Domes, G., Heinrichs, M., Buchel, C., Braus, D.F., Herpertz, S.C., 2007. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol. Psychiatry 62, 11871190. Dominguez, J., Riolo, J.V., Xu, Z., Hull, M.E., 2001. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. J. Neurosci. 21, 349-355. Donaldson, Z.R., Young, L.J., 2009. Oxytocin, vasopressin and the neurogenetics of sociality. Science 322, 900-904. Eaton, R.G., Markowski, V.F., Lumley, L.A., Thompson, J.T., Moses, J., Hull, E.M., 1991. D2 receptors in the paraventricular nucleus regulate genital responses and copulation in male rats. Pharmacol. Biochem. Behav. 39, 177-181. Ebner, K., Bosch, O.J., Krömer, S.A., Singewald, N., Neumann, I.D., 2005. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology 30, 223-230. Enguehard-Gueiffier, C., Hübner, H., El Hakmaoui, A., Allouchi, H., Gmeiner, P., Argiolas, A., Melis, M.R., Gueiffier, A., 2006. 2-[(4-Phenylpiperazin-1-yl)methyl]imidazo(di)azines as selective D4-ligands. Induction of penile erection by 2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo[1,2-a]pyridine (PIP3EA), a potent and selective D4 agonist. J. Med. Chem. 49, 3938-3947. Everitt, B.J., 1990. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses male rats. Neurosci. Biobehav. Rev. 14, 217-232. Fibiger, H.C., Phillips, A.G., 1988. Mesocorticolimbic dopamine system and reward. Ann. N. Y. Acad. Sci. 5, 206-215. French, S.J., Totterdell, S., 2003. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 119, 19-31. 954 M.R. Melis, A. Argiolas / Neuroscience and Biobehavioral Reviews 35 (2011) 939-955 Freund-Mercier, M.J., Richard, P., 1981. Excitatory effects of intraventricular injections of oxytocin on the milk ejection reflex in the rat. Neurosci. Lett. 23, 193-198. Freund-Mercier, M.J., Richard, P., 1984. Electrophysiological evidence for facilitatory control of oxytocin neurons by oxytocin during suckling in the rat. J. Physiol. (Lond.) 352, 447-466. Freund-Mercier, M.J., Stoeckel, M.E., 1995. Somatodendritic autoreceptors on oxytocin neurons. In: Ivell, R., Russel, J.A. (Eds.), Oxytocin, Cellular and Molecular Approaches in Medicine And Research. Adv. Exp. Med. Biol., 365. Plenum Press, New York and London, pp. 185-194. Freund-Mercier, M.J., Stoeckel, M.E., Palacios, J.M., Pazos, J.M., Richard, P.H., Porte, A., 1987. Pharmacological characteristics and anatomical distribution of 3H oxytocin binding sites in the Wistar rat brain studied by autoradiography. Neuroscience 20, 599-614. Giuliano, F., Rampin, O., 2000. Central control of penile erection. Neurosci. Biobehav. Rev. 24, 517-533. Giuliano, F., Allard, J., 2001. Dopamine and sexual function. Int. J. Impotence Res. 13 (Suppl. 3), 18-28. Giuliano, F., Rampin, O., 2004. Neural control of erection. Physiol. Behav. 83, 189-201. Giuliano, F., Bernabè, J., McKenna, K.E., Longueville, F., Rampin, O., 2001. Spinal proerectile effect of oxytocin in anesthetized rats. Am J. Physiol. Regul. Integ. Comp. Physiol. 280, R1870-R1877. Goto, Y., Grace, A.A., 2005. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 8, 805-812. Hawthorn, J., Ang, V.T., Jenkins, J.S., 1985. Effects of lesions in the hypothalamic paraventricular, supraoptic and suprachiasmatic nuclei on vasopressin and oxytocin in rat brain and spinal cord. Brain Res. 346, 51-57. Heier, R.F., Dolak, L.A., Duncan, J.N., Hyslop, D.K., Lipton, M.F., Martin, L.J., Mauragis, M.A., Piercey, M.F., Nichols, N.F., Schreur, P.J., Smith, M.W., Moon, M.W., 1997. Synthesis and biological activities of (R)-5,6-dihydro-N,N-dimethyl-4H-imidazo[4,5,1-ij]quinolin-5-amine) and its metabolites. J. Med. Chem. 40, 639-646. Hsieh, G.C., Hollingsworth, P.R., Martino, B., Chang, R., Terranova, M.A., O’Neill, A.B., Lynch, J.J., Moreland, R.B., Donnelly-Roberts, D.L., Kolasa, T., Mikusa, J.P., McVey, J.M., Marsh, K.C., Sullivan, J.P., Brioni, J.D., 2004. Central mechanisms regulating penile erection in conscious rats: the dopaminergic systems related to the proerectile effect of apomorphine. J. Pharmacol. Exp. Ther. 308, 330-338. Huang, P.L., Dawson, T.M., Bredt, D.S., Snyder, S.H., Fishman, M.C., 1993. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75, 1273-1286. Huber, D., Veinante, P., Stoop, R., 2005. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245-248. Hull, E.M., Warner, R.K., Bazzett, T.J., Eaton, R.C., Thompson, J.T., 1989. D2/D1 ratio in the medial preoptic area affects copulation of male rats. J. Pharmacol. Exp. Ther. 251, 422-427. Hull, E.M., Du, J., Lorrain, D.S., Matuszewich, L., 1995. Extra-cellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J. Neurosci. 15, 7465-7471. Hull, E.M., Meisel, R.L., Sachs, B.D., 2002. Male sexual behavior. In: Pfaff, D.W., Arnold, A.P., Etgen, A.M., Fahrbach, S.E., Rubin, R.T. (Eds.), Hormones, Brain and Behavior. Academic Press, New York, pp. 3-137. Hurlemann, R., Patin, A., Onur, O.A., Cohen, M.X., Baumgartner, T., Metzler, S., Dziobek, I., Gallinat, J., Wagner, M., Maier, W., Kendrick, K.M., 2010. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 30, 4999-5007. Ivell, R., Russel, J.A., 1995. Oxytocin: Cellular and Molecular Approaches in Medicine and Research. Advances in Experimental Medicine and Biology, vol. 395. Plenum Press, New York. Kelley, A.E., Domesick, V.B., 1982. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience 7, 2321-2335. Kimura, Y., Naitou, Y., Wanibuchi, F., Yamaguchi, T., 2008. 5-HT(2C) receptor activation is a common mechanism on proerectile effects of apomorphine, oxytocin and melanotan-II in rats. Eur. J. Pharmacol. 589, 157-162. Kondo, Y., Sachs, B.D., Sakuma, Y., 1998. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav. Brain Res. 91, 215-222. Kovacs, G.L., Sarnyai, Z., Szabo, G., 1998. Oxytocin and addiction: a review. Psychoneuroendocrinology 23, 945-962. Lee, H.J., Macbeth, A.H., Pagani, J.H., Scott Young 3rd, W., 2009. Oxytocin: the great facilitator of life. Prog. Neurobiol. 88, 127-151. Lindvall, O., Bjorklund, A., Skagerberg, G., 1984. Selective istochemical demonstration of dopamine terminal systems in rat di- and telencephalon: new evidence for dopaminergic innervation of hypothalamic neurosecretory nuclei. Brain Res. 306, 19-30. Liu, Y.C., Salamone, J.D., Sachs, B.D., 1997. Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behav. Neurosci. 111, 1361-1367. Löber, S., Tschammer, N., Hübner, H., Melis, M.R., Argiolas, A., Gmeiner, P., 2009. The azulene framework as a novel bioisostere: design of potent dopamine D4 receptor ligands inducing penile erection. Chem. Med. Chem. 4, 325-328. McCleskey, E.W., Fox, A.P., Feldman, D.H., Cruz, L.J., Olivera, B.M., Tsien, R.W., Yoshikami, D., 1987. _-Conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Prot. Nat. Acad. Sci. U.S.A. 84, 4327-4331. McKenna, K.E., 2000. Some proposals regarding the organization of the central nervous system control of penile erection. Neurosci. Biobehav. Rev. 24, 535-540. Marson, L., McKenna, K.E., 1992. A role for 5-hydroxytryptamine in descending inhibition of spinal sexual reflexes. Exp. Brain Res. 88, 313-318. Marson, L., McKenna, K.E., 1996. CNS cell groups involved in the control of the ischiocavernosus and bulbospongiosus muscles: a transneuronal tracing study using pseudorabies virus. J. Comp. Neurol. 374, 161-179. Meisel, R.L., Sachs, B.D., 1994. The physiology of male sexual behaviour. In: Knobil, E., Neil, J. (Eds.), The Physiology of Reproduction, vol. 2, second edition. Raven Press, New York, pp. 3-96. Melin, P., Kihlstrom, J.E., 1963. Influence of oxytocin on sexual behaviour in male rabbits. Endocrinology 73, 433-435. Melis, M.R., Argiolas, A., 1995a. Dopamine and sexual behaviour. Neurosci. Biobehav. Rev. 19, 19-38. Melis, M.R., Argiolas, A., 1995b. Nitric oxide donors induce penile erection and yawning when injected in the central nervous system of male rats. Eur. J. Pharmacol. 294, 1-9. Melis, M.R., Argiolas, A., 2003. Central oxytocinergic neurotransmission: a drug target for the therapy of psycogenic erectile dysfunction. Curr. Drug Targets 4, 55-66. Melis, M.R., Argiolas, A., Gessa, G.L., 1986. Oxytocin-induced yawning and penile erection: site of action in the brain. Brain Res. 398, 259-265. Melis, M.R., Argiolas, A., Gessa, G.L., 1987. Apomorphine-induced yawning and penile erection: site of action in the brain. Brain Res. 415, 98-104. Melis, M.R., Argiolas, A., Gessa, G.L., 1989a. Apomorphine increases plasma oxytocin levels in rats. Neurosci. Lett. 98, 351-355. Melis, M.R., Argiolas, A., Gessa, G.L., 1989b. Evidence that apomorphine induces penile erection and yawning by releasing oxytocin in the central nervous system. Eur. J. Phamacol. 164, 565-570. Melis, M.R., Argiolas, A., Stancampiano, R., Gessa, G.L., 1990. Effect of apomorphine on oxytocin concentrations in different brain areas and plasma of male rats. Eur. J. Pharmacol. 182, 101-107. Melis, M.R., Mauri, A., Argiolas, A., 1994a. Apomorphine- and oxytocin-induced penile erection and yawning in intact and castrated male rats: effect of sexual steroids. Neuroendocrinology 59, 349-354. Melis, M.R., Stancampiano, R., Argiolas, A., 1994b. Penile erection and yawning induced by paraventricular NMDA injections are mediated by oxytocin. Pharmacol. Biochem. Behav. 48, 203-207. Melis, M.R., Stancampiano, R., Argiolas, A., 1994c. Prevention by NG-nitro-l-arginine methyl ester of apomorphine- and oxytocin-induced penile erection and yawning: site of action in the brain. Pharmacol. Biochem. Behav. 48, 799-804. Melis, M.R., Succu, S., Argiolas, A., 1996. Dopamine agonists increase nitric oxide production in the paraventricular nucleus of the hypothalamus: correlation with penile erection and yawning. Eur. J. Neurosci. 8, 2056-2063. Melis, M.R., Succu, S., Iannucci, U., Argiolas, A., 1997. Oxytocin increases nitric oxide production in the paraventricular nucleus of the hypothalamus: correlation with penile erection and yawning. Reg. Peptides 69, 105-112. Melis, M.R., Succu, S., Mauri, A., Argiolas, A., 1998. Nitric oxide production is increased in the paraventricular nucleus of the hypothalamus of male rats during non-contact penile erections and copulation. Eur. J. Neurosci. 10, 1968-1974. Melis, M.R., Spano, M.S., Succu, S., Argiolas, A., 1999a. The oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin reduces non-contact penile erections in male rats. Neurosci. Lett. 265, 171-174. Melis, M.R., Succu, S., Spano, M.S., Argiolas, A., 1999b. Morphine injected into the paraventricular nucleus of the hypothalamus prevents non-contact erections and impairs copulation: involvement of nitric oxide. Eur. J. Neurosci. 11, 1857-1864. Melis, M.R., Spano, M.S., Succu, S., Argiolas, A., 2000. Effect of excitatory amino acid, dopamine, and oxytocin receptor antagonists on noncontact penile erections and paraventricular nitric oxide production in male rats. Behav. Neurosci. 114, 849-857. Melis, M.R., Succu, S., Mascia, M.S., Cortis, L., Argiolas, A., 2003. Extra-cellular dopamine increases in the paraventricular nucleus of male rats during sexual activity. Eur. J. Neurosci. 17, 1266-1272. Melis, M.R., Succu, S., Mascia, M.S., Argiolas, A., 2004a. Antagonism of cannabinoid CB1receptors in the paraventricular nucleus of male rats induces penile erection. Neurosci. Lett. 359, 17-20. Melis, M.R., Succu, S., Mascia, M.S., Cortis, L., Argiolas, A., 2004b. Extra-cellular excitatory amino acids increase in the paraventricular nucleus of male rats during sexual activity: main role ofNMDAreceptors in erectile function. Eur. J. Neurosci. 19, 2569-2575. Melis, M.R., Succu, S., Mascia, M.S., Argiolas, A., 2005. PD-168,077, a selective dopamine D4 receptor agonist, induces penile erection when injected into the paraventricular nucleus of male rats. Neurosci. Lett. 379, 59-62. Melis, M.R., Succu, S., Mascia, M.S., Sanna, F., Melis, T., Succu, S., Castelli, M.P., Argiolas, A., 2006a. SR 141716A-induced penile erection in male rats: involvement of paraventricular glutamic acid and nitric oxide. Neuropharmacology 50, 219-228. Melis, M.R., Succu, S., Sanna, F., Mascia, M.S., Melis, T., Enguehard-Gueiffier, C., Hubner, H., Gmenier, P., Gueiffier, A., Argiolas, A., 2006b. PIP3EA and PD168077, two selective dopamine D4 receptor agonists, induce penile erection in male rats: site and mechanism of action in the brain. Eur. J. Neurosci. 24, 2021-2030. Melis, M.R., Melis, T., Cocco, C., Succu, S., Sanna, F., Pillolla, G., Boi, A., Ferri, G.L., Argiolas, A., 2007. Oxytocin injected into the ventral tegmental area induces penile erection and increases extra-cellular dopamine in the nucleus accumbens M.R. Melis, A. Argiolas / Neuroscience and Biobehavioral Reviews 35 (2011) 939-955 955 and paraventricular nucleus of the hypothalamus of male rats. Eur. J. Neurosci. 26, 1026-1035. Melis, M.R., Sanna, F., Succu, S., Zarone, P., Boi, A., Argiolas, A., 2009a. The role of oxytocin in the anticipatory and consummatory phases of male rat sexual behaviour. In: Jastrow, H., Feuerbach, D. (Eds.), Handbook of Oxytocin Research: Synthesis, Storage and Release, Actions and Drug forms. Nova Publishers Inc, New York, U.S.A., pp. 109-125. Melis, M.R., Succu, S., Sanna, F., Boi, A., Argiolas, A., 2009b. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine in the nucleus accumbens of male rats. Eur. J. Neurosci. 30, 1349-1357. Melis, M.R., Succu, S., Cocco, C., Caboni, E., Sanna, F., Boi, A., Ferri, G.L., Argiolas, A., 2010. Oxytocin induces penile erection when injected into the ventral subiculum: role of nitric oxide and glutamic acid. Neuropharmacology 58, 1153-1160. Moreland, R.B., Nakane, M., Donnelly-Roberts, D.L., Miller, L.N., Chang, R., Uchic, M.E., Terranova, M.A., Gubbins, E.J., Helfrich, R.J., Namovic, M.T., El-Kouhen, O.F., Masters, J.N., Brioni, J.D., 2004. Comparative pharmacology of human dopamine D(2)-like receptor stable cell lines coupled to calcium flux through Galpha(qo5). Biochem. Pharmacol. 68, 761-772. Moos, F., Freund-Mercier, M.J., Guerne, Y., Guerne, J.M., Stoeckel, M.E., Richard, P., 1984. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J. Endocrinol. 102, 63-72. Murphy, M.R., Seckl, J.R., Burton, S., Checkley, S.A., Lightman, S.L., 1987. Changes in oxytocin and vasopressin secretion during sexual activity in men. J. Clin. Endocrinol. Metab. 65, 738-741. Nishimori, K., Young, L.J., Guo, Q., Wang, Z., Insel, T.R., Matzuk, M.M., 1996. Oxytocin is required for nursing but not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 93, 11699-11704. Patel, S., Freedman, S., Chapman, K.L., Emms, F., Fletcher, A.E., Knowles, M., Marwood, R., Mccallister, G., Myers, J., Curtis, J., Kulagowski, J.J., Leeson, P.D., Ridgill, M., Graham, M., Matheson, S., Rathbone, D., Watt, A.P., Bristow, L.J., Rupniak, N.M., Baskin, E., Lynch, J.J., Ragan, C.I., 1997. Biological profile of L 745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J. Pharmacol. Exp. Ther. 283, 636-647. Pedersen, C.A., Caldwell, J.D., Jirikowski, G.F., Insel, T.R., 1992. Oxytocin in Maternal, Sexual, and Social Behaviors, Annals of the New York Academy of Sciences, vol. 652. The New York Academy of Sciences, New York. Petrovic, P., Kalisch, R., Singer, T., Dolan, R.J., 2008. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J. Neurosci. 28, 6607-6615. Pfaus, J.G., Everitt, B.J., 1995. The psychopharmacology of sexual behavior. In: Knobil, F.E., Kupfer, D.J. (Eds.), Psychopharmacology: the Fourth Generation of Progress. Raven Press, New York, pp. 742-758. Rajfer, J., Aronson, W.J., Bush, P.A., Dorey, F.J., Ignarro, L.J., 1992. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N. Engl. J. Med. 326, 90-94. Roeling, T.A.P., Van Erp, A.M.M., Meelis, W., Kruk, M.R., Veening, J.G., 1991. Behavioural effects of NMDA injected into the hypothalamic paraventricular nucleus of the rat. Brain Res. 550, 220-224. Sachs, B.D., 1997. Erection evoked in male rats by airborne scent from estrous females. Physiol. Behav. 62, 921-924. Sachs, B.D., 2000. Contextual approaches to the physiology and classification of erectile function, erectile dysfunction, and sexual arousal. Neurosci. Biobehav. Rev. 24, 541-560. Sachs, B.D., 2007. A contextual definition of male sexual arousal. Horm. Behav. 51, 569-578. Sanchez, F., Alonso, J.R., Arevalo, R., Blanco, E., Aijon, J., Vazquez, R., 1994. Coexistence of NADPH-diaphorase with vasopressin and oxytocin in the hypothalamic magnocellular neurosecretory nuclei of the rat. Cell Tissue Res. 276, 31-34. Sanna, F., Succu, S., Boi, A., Melis, M.R., Argiolas, A., 2009. Phosphodiesterase type 5 inhibitors facilitate non-contact erections in male rats: site of action in the brain and mechanism of action. J. Sex. Med. 6, 2680-2689. Saphier, D., Feldman, S., 1987. Effects of septal and hippocampal stimuli on paraventricular nucleus neurons. Neuroscience 20, 749-755. Sato-Suzuki, I., Kita, I., Oguri, M., Arita, H., 1998. Stereotyped yawning responses induced by electrical and chemical stimulation of paraventricular nucleus of the rat. J. Neurophysiol. 80, 2765-2775. Schuman, E.M., Madison, D.V., 1994. Nitric oxide and synaptic function. Ann. Rev. Neurosci. 17, 153-183. Snyder, S.H., 1992. Nitric oxide: first in a new class of neurotransmitters? Science 254, 494-496. Sofroniew, M.V., 1983. Vasopressin and oxytocin in the mammalian brain and spinal cord. Trends Neurosci. 6, 467-472. Sokoloff, P., Schwartz, J.C., 1995. Novel dopamine receptors half a decade later. Trends Pharmacol. Sci. 16, 270-275. Southam, E., Garthwaite, J., 1993. The nitric oxide-cyclic GMP signalling pathway in rat brain. Neuropharmacology 32, 1267-1277. Stancampiano, R., Melis, M.R., Argiolas, A., 1994. Penile erection and yawning induced by 5-HT1c agonists in male rats: relationship with dopaminergic and oxytocinergic transmission. Eur. J. Pharmacol. 261, 149-155. Succu, S., Mascia, M.S., Sanna, F., Melis, T., Argiolas, A., Melis, M.R., 2006. The cannabinoid CB1 receptor antagonist SR 141716A induces penile erection by increasing extra-cellular glutamic acid in the paraventricular nucleus of male rats. Behav. Brain Res. 169, 274-281. Succu, S., Sanna, F., Melis, T., Boi, A., Argiolas, A., Melis, M.R., 2007. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: involvement of central oxytocin. Neuropharmacology 52, 1034-1043. Succu, S., Sanna, F., Cocco, C., Melis, T., Boi, A., Ferri, G.L., Argiolas, A., Melis, M.R., 2008. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: role of nitric oxide and cyclic GMP. Eur. J. Neurosci. 28, 813-821. Tanda, G., Pontieri, F.E., Di Chiara, G., 1997. Cannabinoids and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276, 2048-2050. Tang, Y., Rampin, O., Calas, A., Facchinetti, P., Giuliano, F., 1998. Oxytocinergic and serotoninergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience 82, 241-254. Theodosis, D.T., 1985. Oxytocin-immunoreactive terminals synapse on oxytocin neurones in the supraoptic nuclei. Nature (London) 313, 682-684. Tindall, J.S., 1974. Stimuli that cause the release of oxytocin. In: Geiger, S.R., Knobil, E., Sawyer, W.H., Greef, R., Astwood, E.B. (Eds.), Handbook of Physiology. Sect. 7, Endocrinology, vol. IV. American Physiology Society, Washington D.C., pp. 257-267. Torres, G., Lee, S., Rivier, C., 1993. Ontogeny of the rat hypothalamic nitric oxide synthase and colocalization with neuropeptides. Mol. Cell. Neurosci. 4, 155-163. Uhl-Bronner, S., Waltisperger, E., Martinez-Lorenzana, G., Condes, L.M., Freund-Mercier, M.J., 2005. Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience 135, 147-154. Vaccari, C., Lolait, S.J., Ostrowski, N.L., 1998. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology 139, 5015-5033. Van Den Pol, A., 1991. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. J. Neurosci. 11, 2087-2101. Veronneau-Longueville, F., Rampin, O., Freund-Mercier, M.J., Tang, Y., Calas, A., Marson, L., McKenna, K.E., Stoeckel, M.E., Benoit, G., Giuliano, F., 1999. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience 93, 1437-1447. Vincent, S.R., Kimura, H., 1992. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46, 755-784. Wagner, C.K., Clemens, L.G., 1993. Neurophysin-containing pathway from the paraventricular nucleus of the hypothalamus to a sexually dimorphic motor nucleus in lumbar spinal cord. J. Comp. Neurol. 336, 106-116. Winslow, J.T., Insel, T.R., 1991. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J. Neurosci. 11, 2032-2038. Wise, R.A., Rompre, P.-P., 1989. Brain dopamine and reward. Ann. Rev. Psychol. 40, 191-225. Witt, D.M., Insel, T.R., 1994. Male sexual behavior activates c-fos-like protein in oxytocin neurons in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 6, 13-18. Witter, M.P., 2006. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res. 174, 251-264. Woodruff, G.N., Foster, A.C., Gill, R., Kemp, J.A., Wong, E.H., Iversen, L.L., 1987. The interaction between MK-801 and receptors for N-methyl-d-aspartate: functional consequences. Neuropharmacology 26, 903-909. Yamashita, H., Shigeru, O., Inenaga, K., Kasai, M., Uesugi, S., Kannan, H., Kaneko, T., 1987. Oxytocin predominantly excites putative oxytocin neurons in the rat supraoptic nucleus in vitro. Brain Res. 416, 364-368. Yells, D.P., Hendricks, S.E., Prendergast, M.A., 1992. Lesions of the nucleus paragigantocellularis: effects on mating behavior in male rats. Brain Res. 596, 73-79. Young, W.S., Shepard, E., Amico, J., Hennighausen, L., LaMarca, M.E., McKinney, C., Ginns, E.I., 1996. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J. Neuroendocrinol. 8, 847-854. Zahran, A.R., Vachon, P., Courtois, F., Carrier, S., 2000. Increases in intracavernous penile pressure following injections of excitatory amino acid receptor agonists in the hypothalamic paraventricular nucleus of anesthetized rats. J. Urol. 164, 1793-1797.