Comments: Naltrexone is an opioid receptor antagonist used primarily in the management of alcohol dependence and opioid dependence. The article has excellent explanations of the addictive process and behavioral addictions.
by Michael Bostwick, MD and Jeffrey A. Bucci, MD
doi: 10.4065/83.2.226
Mayo Clinic Proceedings, February 2008 vol. 83 no. 2 226-230
Article Outline
Malfunctioning of the brain’s reward center is increasingly understood to underlie all addictive behavior. Composed of mesolimbic incentive salience circuitry, the reward center governs all behavior in which motivation has a central role, including acquiring food, nurturing young, and having sex. To the detriment of normal functioning, basic survival activities can pale in importance when challenged by the allure of addictive substances or behaviors. Dopamine is the neurotransmitter driving both normal and addictive behavior. Other neurotransmitters modulate the amount of dopamine released in response to a stimulus, with the salience determined by the intensity of the dopamine pulse. Opiates (either endogenous or exogenous) exemplify such modulators. Prescribed for treating alcoholism, naltrexone blocks opiates’ capacity to augment dopamine release. This article reviews naltrexone’s mechanism of action in the reward center and describes a novel use for naltrexone in suppressing a euphorically compulsive and interpersonally devastating addiction to Internet pornography.
GABA (γ-aminobutyric acid), ISC (incentive salience circuitry), MAB (motivated adaptive behavior), MRE (motivationally relevant event), NAc (nucleus accumbens), PFC (prefrontal cortex), VTA (ventral tegmental area)
Abstract
Until being overwhelmed by addiction, the mesolimbic reward center serves adaptively to motivate behaviors benefiting both individuals and their species. From deep within the brainstem, it coordinates primal incentives to seek such survival requirements as nourishment, nurture of the young, and sexual contact.1 As addiction develops, other less advantageous rewards become imprinted onto the incentive salience circuitry (ISC) to the detriment of behaviors critical to survival. Increasingly, physicians encounter patients in thrall to addictive behaviors.
As neuroscience further elucidates addiction’s neural underpinnings, it becomes increasingly clear that a malfunctioning reward center is common to all compulsive behaviors, whether drug abuse, overeating, gambling, or excessive sexual activity.2, 3 Although impulsive-compulsive sexual behavior has been little studied,4 it makes intuitive sense that pharmacotherapies effective against one type of addictive behavior would also combat other types. Each behavior has specific triggers and manifestations, yet the final common pathway for all involves neurochemical modulation of dopaminergic activity via receptors in the ventral tegmental area (VTA).3, 5
The VTA has thus become a target for new addiction pharmacotherapies, and naltrexone, an opiate receptor blocker currently approved by the Food and Drug Administration only for alcoholism treatment, is an example of a drug potentially useful to combat multiple addictive behaviors.6 By blocking the capacity of endogenous opioids to trigger dopamine release in response to reward, naltrexone helps extinguish that reward’s addictive power. We present a case of naltrexone prescribed to reduce compulsive Internet use for sexual gratification. Hours the patient spent pursuing cyber-stimulation plummeted, and his psychosocial functioning dramatically improved with the use of naltrexone.
REPORT OF A CASE
The Mayo Clinic Institutional Review Board has approved the reporting of this case.
A male patient first presented to a psychiatrist (J.M.B.) at age 24, with the explanation, “I’m here for sexual addiction. It has consumed my entire life.” He feared losing both marriage and job if he could not contain his burgeoning preoccupation with Internet pornography. He was spending many hours each day chatting online, engaging in extended masturbation sessions, and occasionally meeting cyber-contacts in person for spontaneous, typically unprotected, sex.
Over the next 7 years, the patient dropped repeatedly in and out of treatment. He tried antidepressants, group and individual psychotherapy, Sexual Addicts Anonymous, and pastoral counseling, but not until a naltrexone trial did he sustain success at avoiding compulsive Internet use. When he discontinued naltrexone, his urges returned. When he took naltrexone again, they receded.
From age 10, after discovering his grandfather’s cache of “dirty magazines,” the patient had had a strong appetite for pornography. In his late teens, he engaged in phone sex via credit cards and 900-series commercial telephone connections. Describing himself as a compulsive masturbator, he also subscribed to conservative Christian beliefs. Morally troubled by his own behavior, he claimed his sexual actions emanated—at least in part—from “negative influences from the devil.” After high school, he took an advertising sales job that included overnight travel. Both at work and on trips, he used his computer not only for business-related activities but also for online “cruising” (ie, searching for sexually gratifying activity). Business trips would feature hours of online masturbation and overwhelming urges to visit strip clubs. With 24-hour Internet access at his office, he frequently engaged in all-night online sessions. He quickly developed tolerance, quitting a session only when compelled by exhaustion. Of his sexual addiction, he said, “It was the pit of hell. I got no satisfaction, but I went there anyway.”
Reasoning that the patient might suffer from an obsessive-compulsive disorder variant, his psychiatrist prescribed sertraline at an oral dose of 100 mg/d. Whereas the patient’s mood and self-esteem improved and irritability decreased, an initial decline in sexual urges was not sustained. He stopped taking the sertraline and discontinued his relationship with the psychiatrist for a year.
When the patient finally returned to treatment, he was spending up to 8 hours a day online, masturbating until tissue irritation or fatigue ended the sessions. He had had several “hook-ups” with Internet contacts that included unprotected intercourse and was no longer intimate with his wife for fear of transmitting venereal disease to her. He had lost several jobs as a result of poor productivity from time spent pursuing his compulsions at the expense of work. He described extreme pleasure from the sex itself but equally extreme remorse about his inability to control himself. When sertraline therapy was reinstated, his mood improved, but he still felt “powerless to resist the urges” and again stopped treatment.
When the patient reappeared after another 2-year hiatus, more marital distress, and another lost job, the psychiatrist proposed adding naltrexone to the sertraline therapy. (The sertraline now seemed necessary for an ongoing depressive disorder.) Within a week of treatment with 50 mg/d of oral naltrexone, the patient reported “a measurable difference in sexual urges. I wasn’t being triggered all the time. It was like paradise.” His sense of “overwhelming pleasure” during Internet sessions was much diminished, and he discovered an ability to resist rather than submit to impulses. Not until the naltrexone dose reached 150 mg/d did he report complete control over his impulses. When he tried on his own to taper the drug, he felt it lost its efficacy at 25/d. He went online to test himself, met a potential sexual contact, and reached his car before thinking better of an in-person rendezvous. This time, returning to 50 mg of naltrexone was enough to slake his sexual urges.
In the more than 3 years he has received sertraline and naltrexone, he has been in nearly complete remission from depressive symptoms and compulsive Internet use, as he himself has noted: “I occasionally slip, but I don’t carry it as far, and I have no desire to meet anyone.” As an added benefit, he has discovered that binge drinking has lost its charm. He has had no alcohol in 3 years and has accepted that he “can’t drink without drinking too much.” He remains married, although unhappily so. He has kept the same technology-based job for more than 2 years and is proud of his employment success.
DISCUSSION
For the purpose of this discussion, addiction is defined as compulsive behaviors that persist despite serious negative consequences for personal, social, or occupational function.7 Such behaviors include drug abuse, overeating, restrictive eating, self-mutilation, and excessive gambling.6 They also can be specifically sexual compulsions, including activities or thoughts that we consider this case of excessive Internet use to represent.8 This view of addiction is consistent with behavioral formulations of psychiatric disorders, which assume that all addiction diagnoses are “urge-driven disorders” with compulsive behavior at their core.3, 6 Increased understanding of the neural basis of addiction corroborates this view. Hyman5 calls addiction “a pathological usurpation of the neural mechanisms of learning and memory that under normal circumstances serve to shape survival behaviors related to the pursuit of rewards and the cues that predict them.” It is this neural circuitry of motivated adaptive behavior (MAB)—goal-directed behavior to achieve biologically necessary aims—that addiction subjugates.
In varying guises from traditional static erotic images to videos and chat rooms, the Internet is a growing source of potential sexual titillation and stimulation for many so-called normal people, considerations of the morality—or even definition—of pornography aside. When does the normal use of a substance or an activity for personal gratification become compulsive? With his preoccupation and excessive use as well as the drastic interpersonal and occupational consequences he sustained, the patient described in this case report exemplifies the crossover to the realm of addiction.
An MAB has 2 successive components.9 The first is an activating stimulus motivated by learned associations to an external trigger. That stimulus engenders the second: a goal-directed behavioral response—what Stahl10 calls “a natural high.” Basic MABs include instinctive efforts to locate food, water, sexual contact, and shelter. More complex MABs with psychological overlays include seeking nurturing companionship, social status, or occupational achievement.
The neural network mediating MAB expression (the reward center) is also called ISC, because the value assigned to a stimulus (its salience) determines the incentive (the intensity of the behavioral response the stimulus engenders).5, 11 Incentive salience circuitry components include the VTA, nucleus accumbens (NAc), prefrontal cortex (PFC), and amygdala, each with its particular role in shaping the MAB (Figure). Common to ISC activity in both natural and addictive behaviors is dopamine release into the NAc—so-called priming—in response to impulses from the VTA.3, 5 The dopaminergic projections from VTA to NAc are key ISC elements that interact with glutamatergic projections between all ISC components. The amygdala and PFC provide modulatory input.5 The amygdala assigns a noxious or pleasurable valence—an affective tone—to the stimulus, and the PFC determines the intensity and balance of the behavioral response.9, 12 This pleasure-reward circuitry both alerts the organism when a novel salient stimulus appears and recalls learned associations when a no longer novel but still motivationally relevant stimulus recurs.5, 9, 12
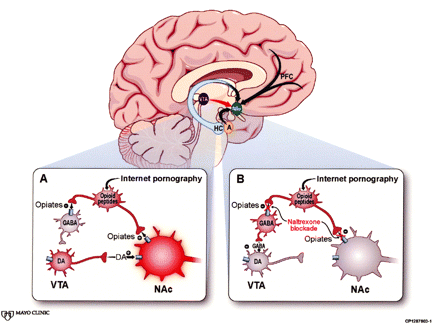
In the cross-sectional image of the brain, incentive salience circuitry (ISC) consists of the ventral tegmental area (VTA) projecting to the nucleus accumbens (NAc). The NAc receives modulatory input from the prefrontal cortex (PFC), amygdala (A), and hippocampus (HC). Box A portrays Internet pornography causing the release of endogenous opioids that enhance dopamine (DA) release in the ISC both directly and indirectly.2 Opiates increase DA action directly through guanine nucleotide-binding protein-coupled opioid receptors on the NAc. They work indirectly on interneurons by binding to opioid receptors that interfere with release of ×-aminobutyric acid (GABA). No longer suppressed by GABA, the VTA sends the NAc an outpouring of DA. Pornography’s salience increases. Box B shows how naltrexone blocks both NAc and interneuron opioid receptors. The DA incentive is no longer enhanced, either directly or indirectly, resulting in pornography’s decreased salience. (Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience,2 copyright 2005.)
The ISC does not function in isolation. Extensive animal studies indicate a pharmacopoeia of neurochemicals originating throughout the cortex and subcortical regions that modulate ISC activation, including endogenous opiodergic, nicotinic, cannabinoid, and other compounds.11, 13 Opiodergic pathways for ISC consist of receptors on the NAc itself that directly interfere with dopamine release2 and of μ-opiate receptors on interneurons that transmit or secrete γ-aminobutyric acid (GABA) and that customarily inhibit dopamine release from VTA dopaminergic neurons.1, 5, 7, 14 When either endogenous opiates (endorphins) or exogenous opiates (morphine and its derivatives) bind to these receptors, GABA release decreases. Opiates prevent interneurons from performing their usual suppressive function, and dopamine levels increase in the VTA.3
All physiologically addictive substances appear to result in faulty ISC activity. Normally at the cellular level, a motivationally relevant event (MRE), such as hunger or sexual arousal, triggers the endogenous opiate release that causes dopamine levels to increase. The ISC responds with an MAB and eventual cellular changes that encode long-term learned associations with the event. These neuroplastic changes cause a more rapid behavioral response when the event recurs, and typically, repeated MRE exposure attenuates and eventually extinguishes VTA dopamine release. Dopamine release is no longer necessary for the organism to perform MABs relevant to survival.
Addictive drugs or activities affect the ISC differently from MREs in that repeated exposures do not extinguish dopamine release.9 Moreover, drugs can outcompete natural stimuli by provoking much more dopamine release for longer periods.5, 9 A vicious addiction cycle results, with ongoing dopamine release ascribing more and more importance to drug-seeking and less and less importance to behaviors basic to normal function and survival.3, 5, 12, 15
The capacity to assign appropriate value to the drug and the ability to resist its siren call—both frontal lobe functions—are deranged in drug addiction.12 “Drug-seeking takes on such power,” writes Hyman, “that it can motivate parents to neglect children, previously law-abiding individuals to commit crimes, and individuals with painful alcohol- or tobacco-related illnesses to keep drinking and smoking.”5 These PFC deficits account for the faulty insight and judgment accompanying these drug-related behaviors.7
Such targeted pharmacotherapies as the morphine-receptor antagonist naltrexone prescribed to our patient can interrupt the unrestrained dopamine crescendo that causes salience attribution and response inhibition functions to become unbalanced. Naltrexone blocks morphine receptors, thereby facilitating an increase in GABA tone and a reduction in NAc dopamine levels through both direct and indirect mechanisms.2 Ultimately, via gradual desensitization, the addictive behavior’s salience should diminish.15, 16
In summary, cellular adaptations in the addict’s PFC result in increased salience of drug-associated stimuli, decreased salience of non-drug stimuli, and decreased interest in pursuing goal-directed activities central to survival. In addition to naltrexone’s approval from the Food and Drug Administration for treating alcoholism, several published case reports have demonstrated its potential for treating pathologic gambling, self-injury, kleptomania, and compulsive sexual behavior.8, 14, 17, 18, 19, 20 We believe this is the first description of its use to combat Internet sexual addiction. Ryback20 specifically studied naltrexone’s efficacy in reducing sexual arousal and hypersexual behavior in adolescents convicted of offenses including rape, bestiality, and sexual activity with young children. While receiving doses between 100 and 200 mg/d, most participants described decreases in arousal, masturbation, and sexual fantasies, as well as increased control over sexual urges.20 Citing evidence from rat studies, Ryback underscores the PFC interplay between dopaminergic and opioid systems, concluding that “a certain endogenous opioid level appears crucial for arousal and sexual functioning.”20
CONCLUSION
The patient had problems stemming both from time wasted in compulsive online masturbatory cybersex and from potential consequences, such as unwanted pregnancy and sexually transmitted diseases, when his virtual activities were extended to extramarital in-person sexual contacts. Adding naltrexone to a medication regimen that already included a selective serotonin reuptake inhibitor coincided with a precipitous decline in and eventual resolution of his addictive symptoms, with a resultant renaissance of his social, occupational, and personal function. With naltrexone occupying morphine receptors on GABAergic interneurons that inhibit VTA dopaminergic neurons, we speculate that endogenous opiate peptides no longer reinforced his compulsive Internet sexual activity. Although he initially continued to crave this activity, as evidenced by his testing behavior, he no longer found it irresistibly rewarding. The salience of the cues prompting Internet sexual activity decreased to the point of the behavior’s near-extinction in the face of his take-it-or-leave-it attitude. Coincidentally but not surprisingly, he found that he no longer enjoyed his binge drinking. More research is needed to confirm that our observations can be generalized to other patients and to clarify the mechanism by which naltrexone extinguishes addictive behavior.
REFERENCES
- Balfour, ME, Yu, L, and Coolen, LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004; 29: 718–730
- Nestler, EJ. Is there a common molecular pathway for addiction?. Nat Neurosci. 2005; 8: 1445–1449
- View in Article
- | CrossRef
- | PubMed
- | Scopus (549)
- View in Article
- | PubMed
- View in Article
- | PubMed
- View in Article
- | CrossRef
- | PubMed
- | Scopus (354)
- View in Article
- | CrossRef
- | PubMed
- View in Article
- | CrossRef
- | PubMed
- | Scopus (272)
- View in Article
- | CrossRef
- | PubMed
- | Scopus (151)
- View in Article
- | CrossRef
- | PubMed
- | Scopus (1148)
- View in Article
- View in Article
- | Abstract
- | Full Text
- | Full Text PDF
- | PubMed
- | Scopus (665)
- View in Article
- | CrossRef
- | PubMed
- | Scopus (1101)
- View in Article
- | CrossRef
- | PubMed
- | Scopus (63)
- View in Article
- | CrossRef
- | PubMed
- | Scopus (51)
- View in Article
- | CrossRef
- | PubMed
- | Scopus (23)
- View in Article
- View in Article
- | CrossRef
- | PubMed
- View in Article
- | CrossRef
- | PubMed
- View in Article
- | PubMed
- | Scopus (245)
- Mick, TM and Hollander, E. Impulsive-compulsive sexual behavior. CNS Spectr. 2006; 11: 944–955
- Grant, JE, Brewer, JA, and Potenza, MN. The neurobiology of substance and behavioral addictions. CNS Spectr. 2006; 11: 924–930
- Hyman, SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005; 162: 1414–1422
- Raymond, NC, Grant, JE, Kim, SW, and Coleman, E. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int Clin Psychopharmacol. 2002; 17: 201–205
- Cami, J and Farre, M. Drug addiction. N Engl J Med. 2003; 349: 975–986
- Grant, JE, Levine, L, Kim, D, and Potenza, MN. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005; 162: 2184–2188
- Kalivas, PW and Volkow, ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005; 162: 1403–1413
- Stahl, SM. in: Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 2nd ed. Cambridge University Press, New York, NY; 2000: 499–537
- Berridge, KC and Robinson, TE. Parsing reward. Trends Neurosci. 2003; 26: 507–513
- Goldstein, RZ and Volkow, ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002; 159: 1642–1652
- Nestler, EJ. From neurobiology to treatment: progress against addiction. Nat Neurosci. 2002; 5: 1076–1079
- Sonne, S, Rubey, R, Brady, K, Malcolm, R, and Morris, T. Naltrexone treatment of self-injurious thoughts and behaviors. J Nerv Ment Dis. 1996; 184: 192–195
- Schmidt, WJ and Beninger, RJ. Behavioural sensitization in addiction, schizophrenia, Parkinson’s disease and dyskinesia. Neurotox Res. 2006; 10: 161–166
- Meyer, JS and Quenzer, LF. Alcohol. in: Psychopharmacology: Drugs, The Brain and Behavior. Sinauer Associates, Inc, Sunderland, MA; 2005: 215–243
- Grant, JE and Kim, SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Ann Clin Psychiatry. 2001; 13: 229–231
- Grant, JE and Kim, SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry. 2002; 63: 349–356
- Kim, SW, Grant, JE, Adson, DE, and Shin, YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001; 49: 914–921
- Ryback, RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004; 65: 982–986